Prioritising the Management of Invasive Non-Native Species
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

11-16589 PRA Record Saperda Candida
PRA Saperda candida 11-16589 (10-15760, 09-15659) European and Mediterranean Plant Protection Organisation Organisation Européenne et Méditerranéenne pour la Protection des Plantes Guidelines on Pest Risk Analysis Decision-support scheme for quarantine pests Version N°3 Pest Risk Analysis for Saperda candida Pest risk analysts: Expert Working group for PRA for Saperda candida (met in 2009-11) ANDERSON Helen (Ms) - The Food and Environment Research Agency (GB) AGNELLO Arthur (Mr) - Department of Entomology New York State Agricultural Experiment Station(USA) BAUFELD Peter (Mr) - Julius Kühn Institut (JKI), Federal Research Centre for Cultivated Plants, Institute for National and International Plant Health (DE) GILL Bruce D. (Mr) - Head Entomology, Ottawa Plant Laboratories, C.F.I.A. (CA) PFEILSTETTER Ernst (Mr) - Julius Kühn Institut (JKI), Federal Research Centre for Cultivated Plants, Institute for National and International Plant Health (DE) (core member) STEFFEK Robert (Mr) - Austrian Agency for Health and Food Safety (AGES), Institute for Plant Health (AT) (core member) VAN DER GAAG Dirk Jan (Mr) - Plant Protection Service (NL) (core member) The Section on risk management was reviewed by the EPPO Panel on Phytosanitary Measures in 2010-02-18. 1 PRA Saperda candida Stage 1: Initiation 1 Identification of a In summer 2008, the presence of Saperda candida was detected for the first time in Germany and in Europe (Nolte & Give the reason for performing the PRA single pest Krieger, 2008). This wood boring insect was observed on the island of Fehmarn on urban trees ( Sorbus intermedia and other host plants) and eradication measures were taken against it. S. candida is considered as a pest of apple trees and other tree species in North America. -

Alien and Invasive Species of Harmful Insects in Bosnia and Herzegovina
Alien and invasive species of harmful insects in Bosnia and Herzegovina Mirza Dautbašić1,*, Osman Mujezinović1 1 Faculty of Forestry University in Sarajevo, Chair of Forest Protection, Urban Greenery and Wildlife and Hunting. Zagrebačka 20, 71000 Sarajevo * Corresponding author: [email protected] Abstract: An alien species – animal, plant or micro-organism – is one that has been introduced as a result of human activity to an area it could not have reached on its own. In cases where the foreign species expands in its new habitat, causing environmental and economic damage, then it is classified as invasive. The maturity of foreign species in the new area may also be influenced by climate changes. The detrimental effect of alien species is reflected in the reduction of biodiversity as well as plant vitality. A large number of alien species of insects are not harmful to plants in new habitats, but there are also those that cause catastrophic consequences to a significant extent. The aim of this paper is to present an overview of newly discovered species of insects in Bosnia and Herzegovina. The research area, for this work included forest and urban ecosystems in the territory of Bosnia and Herzegovina. For the purposes of this paper, an overview of the species found is provided: /HSWRJORVVXVRFFLGHQWDOLV Heidemann; (Hemiptera: Coreidae) - The western conifer seed bug, found at two sites in Central Bosnia, in 2013 and 2015; $UJHEHUEHULGLVSchrank; (Hymenoptera: Argidae) - The berberis sawfly, recorded in one locality (Central Bosnia), in 2015; -

EPPO Reporting Service
ORGANISATION EUROPEENNE EUROPEAN AND ET MEDITERRANEENNE MEDITERRANEAN POUR LA PROTECTION DES PLANTES PLANT PROTECTION ORGANIZATION EPPO Reporting Service NO. 4 PARIS, 2018-04 General 2018/068 New data on quarantine pests and pests of the EPPO Alert List 2018/069 Quarantine lists of Kazakhstan (2017) 2018/070 EPPO report on notifications of non-compliance 2018/071 EPPO communication kits: templates for pest-specific posters and leaflets 2018/072 Useful publications on Spodoptera frugiperda Pests 2018/073 First report of Tuta absoluta in Tajikistan 2018/074 First report of Tuta absoluta in Lesotho 2018/075 First reports of Grapholita packardi and G. prunivora in Mexico 2018/076 First report of Scaphoideus titanus in Ukraine 2018/077 First report of Epitrix hirtipennis in France 2018/078 First report of Lema bilineata in Italy 2018/079 Eradication of Anoplophora glabripennis in Brünisried, Switzerland 2018/080 Update on the situation of Anoplophora glabripennis in Austria Diseases 2018/081 First report of Ceratocystis platani in Turkey 2018/082 Huanglongbing and citrus canker are absent from Egypt 2018/083 Xylella fastidiosa eradicated from Switzerland 2018/084 Update on the situation of Ralstonia solanacearum on roses in Switzerland 2018/085 First report of ‘Candidatus Phytoplasma fragariae’ in Slovenia Invasive plants 2018/086 Ambrosia artemisiifolia control in agricultural areas in North-west Italy 2018/087 Optimising physiochemical control of invasive Japanese knotweed 2018/088 Update on LIFE project IAP-RISK 2018/089 Conference: Management and sharing of invasive alien species data to support knowledge-based decision making at regional level (2018-09-26/28, Bucharest, Romania) 21 Bld Richard Lenoir Tel: 33 1 45 20 77 94 E-mail: [email protected] 75011 Paris Fax: 33 1 70 76 65 47 Web: www.eppo.int EPPO Reporting Service 2018 no. -

Outbreaks of Xylophagous Cerambycid Beetles
Pest specific plant health response plan: Outbreaks of xylophagous cerambycid beetles Figure 1. Adult Saperda candida. © Figure 2. Adult Oemona hirta. © Gilles Gonthier under licence Epitree under licence (https://creativecommons.org/licenses/ (https://creativecommons.org/licen by/2.0/). ses/by-nc/2.0/). 1 © Crown copyright 2019 You may re-use this information (not including logos) free of charge in any format or medium, under the terms of the Open Government Licence. To view this licence, visit www.nationalarchives.gov.uk/doc/open-government-licence/ or write to the Information Policy Team, The National Archives, Kew, London TW9 4DU, or e-mail: [email protected] This document is also available on our website at: https://planthealthportal.defra.gov.uk/pests-and-diseases/contingency-planning/ Any enquiries regarding this document should be sent to us at: The UK Chief Plant Health Officer Department for Environment, Food and Rural Affairs Room 11G32 National Agri-Food Innovation Campus Sand Hutton York YO41 1LZ Email: [email protected] 2 Contents 1. Introduction and scope .............................................................................................. 4 2. Summary of threat ...................................................................................................... 4 3. Response .................................................................................................................... 4 Official action to be taken following the suspicion or confirmation of a cerambycid on imported -

Developing a List of Invasive Alien Species Likely to Threaten Biodiversity and Ecosystems in the European Union
Received: 25 July 2018 | Accepted: 7 November 2018 DOI: 10.1111/gcb.14527 PRIMARY RESEARCH ARTICLE Developing a list of invasive alien species likely to threaten biodiversity and ecosystems in the European Union Helen E. Roy1 | Sven Bacher2 | Franz Essl3,4 | Tim Adriaens5 | David C. Aldridge6 | John D. D. Bishop7 | Tim M. Blackburn8,9 | Etienne Branquart10 | Juliet Brodie11 | Carles Carboneras12 | Elizabeth J. Cottier-Cook13 | Gordon H. Copp14,15 | Hannah J. Dean1 | Jørgen Eilenberg16 | Belinda Gallardo17 | Mariana Garcia18 | Emili García‐Berthou19 | Piero Genovesi20 | Philip E. Hulme21 | Marc Kenis22 | Francis Kerckhof23 | Marianne Kettunen24 | Dan Minchin25 | Wolfgang Nentwig26 | Ana Nieto18 | Jan Pergl27 | Oliver L. Pescott1 | Jodey M. Peyton1 | Cristina Preda28 | Alain Roques29 | Steph L. Rorke1 | Riccardo Scalera18 | Stefan Schindler3 | Karsten Schönrogge1 | Jack Sewell7 | Wojciech Solarz30 | Alan J. A. Stewart31 | Elena Tricarico32 | Sonia Vanderhoeven33 | Gerard van der Velde34,35,36 | Montserrat Vilà37 | Christine A. Wood7 | Argyro Zenetos38 | Wolfgang Rabitsch3 1Centre for Ecology & Hydrology, Wallingford, UK 2University of Fribourg, Fribourg, Switzerland 3Environment Agency Austria, Vienna, Austria 4Division of Conservation Biology, Vegetation Ecology and Landscape Ecology, University Vienna, Vienna, Austria 5Research Institute for Nature and Forest (INBO), Brussels, Belgium 6Department of Zoology, University of Cambridge, Cambridge, UK 7The Laboratory, The Marine Biological Association, Plymouth, UK 8University College London, -

(Coleoptera) of the Huron Mountains in Northern Michigan
The Great Lakes Entomologist Volume 19 Number 3 - Fall 1986 Number 3 - Fall 1986 Article 3 October 1986 Ecology of the Cerambycidae (Coleoptera) of the Huron Mountains in Northern Michigan D. C. L. Gosling Follow this and additional works at: https://scholar.valpo.edu/tgle Part of the Entomology Commons Recommended Citation Gosling, D. C. L. 1986. "Ecology of the Cerambycidae (Coleoptera) of the Huron Mountains in Northern Michigan," The Great Lakes Entomologist, vol 19 (3) Available at: https://scholar.valpo.edu/tgle/vol19/iss3/3 This Peer-Review Article is brought to you for free and open access by the Department of Biology at ValpoScholar. It has been accepted for inclusion in The Great Lakes Entomologist by an authorized administrator of ValpoScholar. For more information, please contact a ValpoScholar staff member at [email protected]. Gosling: Ecology of the Cerambycidae (Coleoptera) of the Huron Mountains i 1986 THE GREAT LAKES ENTOMOLOGIST 153 ECOLOGY OF THE CERAMBYCIDAE (COLEOPTERA) OF THE HURON MOUNTAINS IN NORTHERN MICHIGAN D. C. L Gosling! ABSTRACT Eighty-nine species of Cerambycidae were collected during a five-year survey of the woodboring beetle fauna of the Huron Mountains in Marquette County, Michigan. Host plants were deteTITIined for 51 species. Observations were made of species abundance and phenology, and the blossoms visited by anthophilous cerambycids. The Huron Mountains area comprises approximately 13,000 ha of forested land in northern Marquette County in the Upper Peninsula of Michigan. More than 7000 ha are privately owned by the Huron Mountain Club, including a designated, 2200 ha, Nature Research Area. The variety of habitats combines with differences in the nature and extent of prior disturbance to produce an exceptional diversity of forest communities, making the area particularly valuable for studies of forest insects. -
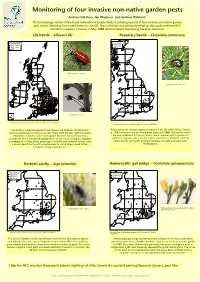
Monitoring of Four Invasive Non-Native Garden Pests
Monitoring of four invasive non-native garden pests Andrew Salisbury, Ian Waghorn and Andrew Halstead The Entomology section of the Royal Horticultural Society (RHS) is collating records of four invasive non-native garden pest insects following their establishment in the UK. Data collection has previously relied on data gathered from RHS members’ enquiries, however in May 2008 internet- based monitoring has been launched. Lily beetle – Lilioceris lilii Rosemary beetle – Chrysolina americana First reported Firs t rec orded 1994-1999 1939-1989 2000-2004 1990-1999 2005- 2000+ Adult lily beetle (Photo R. Key) Rosemary beetle adult (RHS) Lily beetle larvae (RHS) Distribution of the Lily beetle in Britain and Ireland (December 2007). Produced using DMAP© Distribution of Rosemary beetle in Britain. (January 2008). Produced using DMAP© Lily beetle is a defoliating pest of lilies (Lilium) and fritillaries (Fritillaria) that Rosemary beetle was first reported outdoors in the UK at RHS Wisley Garden became established in Surrey in the late 1930s. Until the late 1980s the beetle in 1994, however it was not found there again until 2000. During that time it remained confined to south east England. However during the past two became established in London, and is now a common pest in gardens in decades the beetle has spread rapidly and it is now found throughout England, south east England, with scattered reports from the rest of Britain. Both the is widespread in Wales and is spreading in Scotland and Northern Ireland. Little adults and the grey grubs defoliate rosemary, lavender and some other is known about the threat this beetle poses to native Snake’s head fritillary related plants. -

Oemona Hirta (Revised)
EUROPEAN AND MEDITERRANEAN PLANT PROTECTION ORGANIZATION ORGANISATION EUROPEENNE ET MEDITERRANEENNE POUR LA PROTECTION DES PLANTES 15-21045 Pest Risk Analysis for Oemona hirta (revised) September 2014 EPPO 21 Boulevard Richard Lenoir 75011 Paris www.eppo.int [email protected] This risk assessment follows the EPPO Standard PM PM 5/3(5) Decision-support scheme for quarantine pests (available at http://archives.eppo.int/EPPOStandards/pra.htm) and uses the terminology defined in ISPM 5 Glossary of Phytosanitary Terms (available at https://www.ippc.int/index.php). This document was first elaborated by an Expert Working Group and then reviewed by the Panel on Phytosanitary Measures and if relevant other EPPO bodies. Cite this document as: EPPO (2014) Revised Pest risk analysis for Oemona hirta. EPPO, Paris. Available at http://www.eppo.int/QUARANTINE/Pest_Risk_Analysis/PRA_intro.htm Photo:Adult Oemona hirta. Courtesy Prof. Qiua Wang, Institute of Natural Resources, Massey University (NZ) 15-21045 (13-19036, 13-18422, 12-18133) Pest Risk Analysis for Oemona hirta This PRA follows the EPPO Decision-support scheme for quarantine pests PM 5/3 (5). A preliminary draft has been prepared by the EPPO Secretariat and served as a basis for the work of an Expert Working Group that met in the EPPO Headquarters in Paris on 2012-05-29/06-01. This EWG was composed of: Mr John Bain, Scion Forest Protection (New Zealand Forest Research Institute Ltd.), Rotorua, New Zealand Dr Dominic Eyre, Food and Environment Research Agency, York, UK Dr Hannes Krehan, Federal Office, Vienna Institute of Forest Protection, Vienna, Austria Dr Panagiotis Milonas, Benaki Phytopathological Institute, Kifissia, Greece Dr Dirkjan van der Gaag, Plant Protection Service, Wageningen, Netherlands Dr Qiao Wang, Massey University, Palmerston North, New Zealand. -

PRA on Apriona Species
EUROPEAN AND MEDITERRANEAN PLANT PROTECTION ORGANIZATION ORGANISATION EUROPEENNE ET MEDITERRANEENNE POUR LA PROTECTION DES PLANTES 16-22171 (13-18692) Only the yellow note is new compared to document 13-18692 Pest Risk Analysis for Apriona germari, A. japonica, A. cinerea Note: This PRA started 2011; as a result, three species of Apriona were added to the EPPO A1 List: Apriona germari, A. japonica and A. cinerea. However recent taxonomic changes have occurred with significant consequences on their geographical distributions. A. rugicollis is no longer considered as a synonym of A. germari but as a distinct species. A. japonica, which was previously considered to be a distinct species, has been synonymized with A. rugicollis. Finally, A. cinerea remains a separate species. Most of the interceptions reported in the EU as A. germari are in fact A. rugicollis. The outcomes of the PRA for these pests do not change. However A. germari has a more limited and a more tropical distribution than originally assessed, but it is considered that it could establish in Southern EPPO countries. The Panel on Phytosanitary Measures agreed with the addition of Apriona rugicollis to the A1 list. September 2013 EPPO 21 Boulevard Richard Lenoir 75011 Paris www.eppo.int [email protected] This risk assessment follows the EPPO Standard PM PM 5/3(5) Decision-support scheme for quarantine pests (available at http://archives.eppo.int/EPPOStandards/pra.htm) and uses the terminology defined in ISPM 5 Glossary of Phytosanitary Terms (available at https://www.ippc.int/index.php). This document was first elaborated by an Expert Working Group and then reviewed by the Panel on Phytosanitary Measures and if relevant other EPPO bodies. -

EPPO Reporting Service
ORGANISATION EUROPEENNE EUROPEAN AND ET MEDITERRANEENNE MEDITERRANEAN POUR LA PROTECTION DES PLANTES PLANT PROTECTION ORGANIZATION EPPO Reporting Service NO. 8 PARIS, 2017-08 General 2017/145 New data on quarantine pests and pests of the EPPO Alert List 2017/146 Quarantine list of the Eurasian Economic Union (EAEU) 2017/147 EPPO communication kits: new templates for pest-specific posters and leaflets Pests 2017/148 Rhynchophorus ferrugineus does not occur in Australia 2017/149 Platynota stultana (Lepidoptera: Tortricidae): added again to the EPPO Alert List Diseases 2017/150 First report of Puccinia hemerocallidis in Portugal 2017/151 First report of Pantoea stewartii in Malaysia 2017/152 Citrus leprosis disease is associated with several viruses 2017/153 Brevipalpus phoenicis, vector of citrus leprosis, is a species complex Invasive plants 2017/154 The suppressive potential of some grass species on the growth and development of Ambrosia artemisiifolia 2017/155 Bidens subalternans in the EPPO region: addition to the EPPO Alert List 2017/156 Abiotic constraints and biotic resistance control the establishment success of Humulus scandens 21 Bld Richard Lenoir Tel: 33 1 45 20 77 94 E-mail: [email protected] 75011 Paris Fax: 33 1 70 76 65 47 Web: www.eppo.int EPPO Reporting Service 2017 no. 8 - General 2017/145 New data on quarantine pests and pests of the EPPO Alert List By searching through the literature, the EPPO Secretariat has extracted the following new data concerning quarantine pests and pests included (or formerly included) on the EPPO Alert List, and indicated in bold the situation of the pest concerned using the terms of ISPM no. -

Contribution to the Knowledge of Sawfly Fauna (Hymenoptera, Symphyta) of the Low Tatras National Park in Central Slovakia Ladislav Roller - Karel Beneš - Stephan M
NATURAE TUTELA 10 57-72 UITOVSKY MIM li AS 'nix, CONTRIBUTION TO THE KNOWLEDGE OF SAWFLY FAUNA (HYMENOPTERA, SYMPHYTA) OF THE LOW TATRAS NATIONAL PARK IN CENTRAL SLOVAKIA LADISLAV ROLLER - KAREL BENEŠ - STEPHAN M. BLANK JAROSLAV HOLUŠA - EWALD JANSEN - M ALTE JÄNICKE- SIGBERT KALUZA - ALEXANDRA KEHL - INGA KEHR - MANFRED KRAUS - ANDREW D. LISTON - TOMMI NYMAN - HAIYAN NIE - HENRI SAVINA - ANDREAS TAEGER - MEICAI WEI L. Roller, K. Beneš, S. M. Blank, J. Iloluša, E. Jansen, M. Jänicke, S. Kaluza, A. Kehl, 1. Kehr, M. Kraus, A. D. Liston,T. Nyman, II. Nie, II. Savina, A. Taeger, M. Wei: Príspevok k poznaniu fauny hrubopásych (Hymenoptera, Symphyta) Národného parku Nízke Tatry Abstrakt: Počas deviateho medzinárodného pracovného stretnutia špecialistov na hrubopáse blanokrídlovce (Hymenoptera, Symphyta) v júni 2005 bol vykonaný ľaunistický prieskum hrubopásych na viacerých lokalitách Národného parku Nízke Tatry. Celkovo bolo zaznamenaných 200 druhov a osem čeľadí hrubopásych. Ďalších dvanásť taxónov nebolo identifikovaných do druhu. Dolerus altivolus, D. hibernicus, Eriocampa dorpatica, Euura hastatae, Fenella monilicornis, Nematus yokohamensis tavastiensis, Pachynematus clibrichellus. Phyllocolpa excavata, P. polita, P. ralieri, Pontania gallarum, P. virilis, Pristiphora breadalbanensis, P. coactula a Tenthredo ignobilis boli zistené na Slovensku po prvýkrát. Významný počet ľaunistický zaujímavých nálezov naznačuje vysokú zachovalosť študovaných prírodných stanovíšť v širšom okolí Demänovskej a Jánskej doliny, Svarína a v národnej prírodnej rezervácii Turková. Kľúčové slová: Hymenoptera, Symphyta, Národný park Nízke Tatry, ľaunistický výskum INTRODUCTION Sawflies are the primitive phytophagous hymenopterans (suborder Symphyta), represented in Europe by 1366 species (LISTON, 1995; TAEGER & BLANK, 2004; TAEGER et al., 2006). In Slovakia, about 650 species belonging to 13 families of the Symphyta should occur (ROLLER, 1999). -

Hymenoptera, Symphyta: Argidae Und Cimbicidae)
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Linzer biologische Beiträge Jahr/Year: 1996 Band/Volume: 0028_2 Autor(en)/Author(s): Schwarz Martin, Blank Stephan M. Artikel/Article: Bürst- und Keulhornblattwespen im Bundesland Salzburg (Österreich) (Hymenoptera, Symphyta: Argidae und Cimbicidae). 937-951 © Biologiezentrum Linz/Austria; download unter www.biologiezentrum.at Linzer biol. Beitr. 28/2 937-951 31.12.1996 Bürsten- und Keulhornblattwespen im Bundesland Salzburg (Österreich) (Hymenoptera, Symphyta: Argidae und Cimbicidae) M. SCHWARZ & S.M. BLANK Abstract: Argidae and Cimbicidae in the province of Salzburg (Austria) (Hymenoptera, Symphyta). Available material of Argidae and Cimbicidae collected in Salzburg (Austria), and ad- ditional published data are summarized and evaluated. Presently 16 species of Argidae and 14 species of Cimbicidae are known to occur in this province. 15 species (6 Argidae, 8 Cimbicidae) are recorded for the first time. Aprosthema tardum (KLUG 1814) (species revocata) and A. melanura (KLUG 1814) are characterized. Einleitung Die Pflanzenwespenfauna des Bundeslandes Salzburg ist sehr lückenhaft bekannt. In der Literatur finden sich nur wenige faunistische Angaben über Argidae oder Cim- bicidae. STORCH (1868) erwähnt 18 Arten aus dem Gebiet, ohne genauere Funddaten anzuführen. Die Angaben von Storch wurden von SCHEDL (1982) in den Catalogus Faunae Austriae übernommen, sie gelten jedoch als sehr unzuverlässig (EMBACHER 1990, SCHWARZ 1994). Einzelne weitere Nachweise aus Salzburg werden bei FRANZ (1982) und GlRAUD (1858) aufgeführt. Die Hymenopterensammlung von Dr. P.P. BABIY (1894-1989), die größtenteils im Haus der Natur in Salzburg aufbewahrt wird, enthält derzeit die umfangreichste Aufsammlung von Pflanzenwespen von Salzburg. Neueres Material wurde vor allem von J.