And Description of Two New Species of the Cyrtochilum Divaricatum-Alliance from Colombia Dariusz L
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ORCHIDACEAE: ONCIDIINAE) and a SOLUTION to a TAXONOMIC CONUNDRUM Lankesteriana International Journal on Orchidology, Vol
Lankesteriana International Journal on Orchidology ISSN: 1409-3871 [email protected] Universidad de Costa Rica Costa Rica Dalström, Stig NEW COMBINATIONS IN ODONTOGLOSSUM (ORCHIDACEAE: ONCIDIINAE) AND A SOLUTION TO A TAXONOMIC CONUNDRUM Lankesteriana International Journal on Orchidology, vol. 12, núm. 1, abril, 2012, pp. 53-60 Universidad de Costa Rica Cartago, Costa Rica Available in: http://www.redalyc.org/articulo.oa?id=44339823005 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative LANKESTERIANA 12(1): 53—60. 2012. NEW COMBINATIONS IN ODONTOGLOSSUM (ORCHIDACEAE: ONCIDIINAE) AND A SOLUTION TO A TAXONOMIC CONUNDRUM STIG DALSTRÖM 2304 Ringling Boulevard, unit 119, Sarasota FL 34237, U.S.A. Research Associate: Lankester Botanical Garden, University of Costa Rica and Andean Orchids Research Center, University Alfredo Pérez Guerrero, Ecuador National Biodiversity Centre, Serbithang, Thimphu, Bhutan [email protected] ABSTRACT. The diminutively flowered Oncidium koechliniana demonstrates a unique combination of features that justifies a transfer of it and all here accepted species in closely related genera Cochlioda and Solenidiopsis to Odontoglossum, which is executed here. Distinguishing features to separate Odontoglossum from Oncidium are based on geographic distribution, and flower morphology, which is demonstrated with illustrations. RESUMEN. Oncidium koechliniana, de flores diminutas, presenta una combinacíon de características únicas que justifica su transferencia, así como de todas las especies aquí aceptadas de los génerosCochlioda y Solenidiopsis a Odontoglossum, transferencias que se hacen en este artículo. La características distintiva para separar Odontoglossum de Oncidium están basadas en distribución geográfica y morfología floral, que se muestran a través de ilustraciones. -

Epilist 1.0: a Global Checklist of Vascular Epiphytes
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2021 EpiList 1.0: a global checklist of vascular epiphytes Zotz, Gerhard ; Weigelt, Patrick ; Kessler, Michael ; Kreft, Holger ; Taylor, Amanda Abstract: Epiphytes make up roughly 10% of all vascular plant species globally and play important functional roles, especially in tropical forests. However, to date, there is no comprehensive list of vas- cular epiphyte species. Here, we present EpiList 1.0, the first global list of vascular epiphytes based on standardized definitions and taxonomy. We include obligate epiphytes, facultative epiphytes, and hemiepiphytes, as the latter share the vulnerable epiphytic stage as juveniles. Based on 978 references, the checklist includes >31,000 species of 79 plant families. Species names were standardized against World Flora Online for seed plants and against the World Ferns database for lycophytes and ferns. In cases of species missing from these databases, we used other databases (mostly World Checklist of Selected Plant Families). For all species, author names and IDs for World Flora Online entries are provided to facilitate the alignment with other plant databases, and to avoid ambiguities. EpiList 1.0 will be a rich source for synthetic studies in ecology, biogeography, and evolutionary biology as it offers, for the first time, a species‐level overview over all currently known vascular epiphytes. At the same time, the list represents work in progress: species descriptions of epiphytic taxa are ongoing and published life form information in floristic inventories and trait and distribution databases is often incomplete and sometimes evenwrong. -

Oncidiinae: Orchidaceae) from PERU Lankesteriana International Journal on Orchidology, Vol
Lankesteriana International Journal on Orchidology ISSN: 1409-3871 [email protected] Universidad de Costa Rica Costa Rica Dalström, Stig; Deburghgraeve, Guido; Ruíz Perez, Saul THREE NEW SHOWY BUT ENDANGERED CYRTOCHILUM SPECIES (Oncidiinae: Orchidaceae) FROM PERU Lankesteriana International Journal on Orchidology, vol. 12, núm. 2, agosto, 2012, pp. 93- 99 Universidad de Costa Rica Cartago, Costa Rica Available in: http://www.redalyc.org/articulo.oa?id=44339824001 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative LANKESTERIANA 12(2): 93—99. 2012. THrEE NEW sHoWY BUT ENDANGErED CYRTOCHILUM sPECIEs (oNCIDIINAE: orCHIDACEAE) FroM PErU STIG DALSTRÖM1,4, GUIDO DEBURGHGRAEVE2 & SAUL RUÍZ PEREZ3 1 2304 Ringling Boulevard, unit 119, Sarasota FL 34237, USA Research Associate, Lankester Botanical Garden, University of Costa Rica, Cartago, Costa Rica and National Biodiversity Centre, Serbithang, Bhutan 2 Meersstraat 147, 1770 Liedekerke, Belgium 3 Allamanda 142, Surco, Lima 33, Peru 4 Corresponding author: [email protected] ABSTRACT. Three new Cyrtochilum species from Peru that are endangered by habitat destruction, are here described, illustrated and compared with similar species. KEY WORDS: Cyrtochilum, endangered species, Orchidaceae, Oncidiinae, new species, Peru, taxonomy The genus Cyrtochilum Kunth has gone through creeping on a bracteate rhizome, oblong ovoid, ca. quite a taxonomic turmoil during its two centuries 10 × 5 cm, distantly bifoliate (terminal leaf ca. 2 long history. The trouble has mainly been caused cm above lower leaf), surrounded basally by 7-8 by difficulties in defining the genus based on floral distichous sheaths, the uppermost foliaceous. -

Redalyc.ONCIDIUM SURPRISES with DEOXYRIBONUCLEIC ACID
Lankesteriana International Journal on Orchidology ISSN: 1409-3871 [email protected] Universidad de Costa Rica Costa Rica ZELENKO, HARRY ONCIDIUM SURPRISES WITH DEOXYRIBONUCLEIC ACID Lankesteriana International Journal on Orchidology, vol. 7, núm. 1-2, marzo, 2007, pp. 458-460 Universidad de Costa Rica Cartago, Costa Rica Available in: http://www.redalyc.org/articulo.oa?id=44339813094 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative LANKESTERIANA 7(1-2): 458-460. 2007. ONCIDIUM SURPRISES WITH DEOXYRIBONUCLEIC ACID HARRY ZELENKO Asociación de Orquideología de Quito and Greater New York Orchid Society P.O. Box 17-22-20043 Cumbaya, Quito, Ecuador [email protected] There is an armchair taxonomist I know that wrote Another monotype, Oncidium onustum, was studied that the taxonomy of the Oncidium alliance was a mess. by Williams and Chase and with DNA research and I’d been thinking that the remark was based on lack of other observations, they confirmed that because there knowledge. In my opinion, he is way off base. Know were a number of differences with this species, it was that I am not a taxonomist… only a grower and an removed from the body of Oncidium and it is now artist. But I do believe that the Oncidium alliance is a called Zelenkoa onusta. They grow on cacti as well as reasonably well organized taxonomy. trees in southern Ecuador and northern Peru. -

Caucaea Colombiana Uribe-Velez, Sauleda & Szlachetko an Addition
ISSN 2325-4785 New World Orchidaceae – Nomenclatural Notes Nomenclatural Note – Issue No. 80 August 18, 2020. Caucaea colombiana Uribe-Velez, Sauleda & Szlachetko an Addition to the Flora of Colombia. Carlos Uribe-Velez1, Ruben P. Sauleda2 and Dariusz L. Szlachetko3 1Calle 115 #5-23 Bogota, Colombia. 26442 SW 107 Ct. Miami, Fl, 33173. 3Department of Plant Taxonomy and Nature Conservation, University of Gdańsk, Wita Stwosza 59, 80-308 Gdańsk, Poland. Abstract A new species of Caucaea Schltr. from Norte de Santander, Colombia is described. Kraenzlin (1922) originally included the species now included in the genus Caucaea in Oncidium sect. Cucullata Kraenzlin, typified by Oncidium cucullatum Lindl. This was the first comprehensive infrageneric classification of the genus Oncidium. Stacy (1975) suggested that the species included in this section were distinctive from Oncidium. Molecular studies (Williams et al., 2001) of some of the species included in Kraenzlin’s sect. Cucullata suggest a close relation with what was the monotypic genus Caucaea Schltr., typified by Caucaea radiata (Lindl.) Schltr. Williams et al. (2001) suggested transferring the species of the sect. Cucullata to Caucaea. This concept was rejected by Dodson and Luer (2005). However, studies of gynostemium morphology (Szlachetko & Mytnik-Ejsmont, 2009) confirm the similarity of the reproductive structures between C. radiata and species of the sect. Cucullata. In addition, subsequent genetic analysis of the Oncidiinae by Neubig et al. (2012) confirmed the molecular analysis of Williams et al. (2001) demonstrating a close phylogenetic relation between C. radiata and species of the sect. Cucullata. The analysis of Williams et al. (2001) also demonstrates a clear separation between the species of Oncidium but, a close relation between species of the sect Cucullata and taxa of the genus Cyrtochilum complex. -

University of Florida Thesis Or Dissertation Formatting
A MONOGRAPH OF THE GENUS LOCKHARTIA (ORCHIDACEAE: ONCIDIINAE) By MARIO ALBERTO BLANCO-COTO A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY UNIVERSITY OF FLORIDA 2011 1 © 2011 Mario Alberto Blanco-Coto 2 To my parents, who have always supported and encouraged me in every way. 3 ACKNOWLEDGMENTS Many individuals and institutions made the completion of this dissertation possible. First, I thank my committee chair, Norris H. Williams, for his continuing support, encouragement and guidance during all stages of this project, and for providing me with the opportunity to visit and do research in Ecuador. W. Mark Whitten, one of my committee members, also provided much advice and support, both in the lab and in the field. Both of them are wonderful sources of wisdom on all matters of orchid research. I also want to thank the other members of my committee, Walter S. Judd, Douglas E. Soltis, and Thomas J. Sheehan for their many comments, suggestions, and discussions provided. Drs. Judd and Soltis also provided many ideas and training through courses I took with them. I am deeply thankful to my fellow lab members Kurt Neubig, Lorena Endara, and Iwan Molgo, for the many fascinating discussions, helpful suggestions, logistical support, and for providing a wonderful office environment. Kurt was of tremendous help in the lab and with Latin translations; he even let me appropriate and abuse his scanner. Robert L. Dressler encouraged me to attend the University of Florida, provided interesting discussions and insight throughout the project, and was key in suggesting the genus Lockhartia as a dissertation subject. -

2017 Adamčík, Slavomír, Jančovičová, Soňa, Looney, Brian P, Adamčíková, Katarína, Birkebak, Joshua M, Moreau, Pierre-Arthur, Matheny, P Brandon
2017 Adamčík, Slavomír, Jančovičová, Soňa, Looney, Brian P, Adamčíková, Katarína, Birkebak, Joshua M, Moreau, Pierre-Arthur, Matheny, P Brandon. (2017). Circumscription of species in the Hodophilus foetens complex (Clavariaceae, Agaricales) in Europe. Mycological Progress, 16(1): 47–62. Antonelli, Alexandre, Hettling, Hannes, Condamine, Fabien L., Vos, Karin, Nilsson, R. Henrik, Sanderson, Michael J, Sauquet, Hervé, Scharn, Ruud, Silvestro, Daniele, Töpel, Mats H., Bacon, Christine D., Oxelman, Bengt & Vos, Rutger A (2017). Toward a Self-Updating Platform for Estimating Rates of Speciation and Migration, Ages, and Relationships of Taxa. Systematic biology, 66(2: 152–166. Athanasiadis, Athanasios. (2017). A study of the original material of Lithothamnion engelhartii Foslie (Corallinales, Rhodophyta). Botanica Marina, 60(1): 67–78. Athanasiadis, A. (2017). Capensia fucorum (Esper) gen. et comb. nov. (Mesophyllaceae, Corallinales, Rhodophyta): a hemiparasite on Gelidium from South Africa. Botanica Marina. 60(5): 555–565. Boltenkov, Eugeny V, & Govaerts, Rafael. (2017). Typification of names and nomenclatural notes on juno irises (Iridaceae) from Western Asia, Western Europe, and North Africa. Phytotaxa, 303(2): 125–140. Claudel, C., Buerki, S., Chatrou, L. W., Antonelli, Alexandre, Alvarez, N. & Hetterscheid, W. (2017). Large-scale phylogenetic analysis of Amorphophallus (Araceae) derived from nuclear and plastid sequences reveals new subgeneric delineation. Botanical Journal of the Linnean Society, 184(1): 32–45 Condamine, Fabien L., Leslie, Andrew B. & Antonelli, Alexandre (2017). Ancient islands acted as refugia and pumps for conifer diversity. Cladistics. 33: 1. Díaz-Tapia, Pilar, McIvor, Lynne, Freshwater, D Wilson, Verbruggen, Heroen, Wynne, Michael J, & Maggs, Christine A. (2017). The genera Melanothamnus Bornet & Falkenberg and Vertebrata SF Gray constitute well–defined clades of the red algal tribe Polysiphonieae (Rhodomelaceae, Ceramiales). -
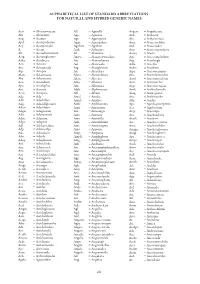
Orchid Name Abbreviations List
ALPHABETICAL LIST OF STANDARD ABBREVIATIONS FOR NATURAL AND HYBRID GENERIC NAMES Acw. = Aberconwayara All. = Aganella Angcst. = Angulocaste Abr. = Aberrantia Agn. = Aganisia Ank. = Anikaara Acp. = Acampe Agt. = Aganopeste Akr. = Ankersmitara Apd. = Acampodorum Agsp. = Agasepalum Anct. = Anoectochilus Acy. = Acampostylis Agubata = Agubata Atd. = Anoectodes A. = Aceras Aitk. = Aitkenara Ano. = Anoectogoodyera Ah. = Acerasherminium Al. = Alamania Anota = Anota Actg. = Aceratoglossum Agwa. = Alangreatwoodara Ayp. = Ansecymphyllum Acba. = Acinbreea Atc. = Alantuckerara Asg. = Anselangis Acn. = Acineta Aat. = Alaticaulia Aslla. = Ansellia Ain. = Acinopetala Atg. = Alatiglossum Asdm. = Ansidium Aip. = Aciopea Alc. = Alcockara Arpt. = Anteriocamptis Akm. = Ackermania Alxra. = Alexanderara Ahc. = Anterioherorchis Aks. = Ackersteinia Alcra. = Aliceara Atml. = Anteriomeulenia Aco. = Acoridium Alna. = Allenara Antr. = Anteriorchis Apa. = Acrolophia Aln. = Allioniara Atsp. = Anterioserapias Aro. = Acronia Alph. = Alphonsoara Anth. = Anthechostylis Acro. = Acropera Alv. = Alvisia Antg. = Antheglottis Ada = Ada Amal. = Amalia Anr. = Antheranthe Adh. = Adachilum Amals. = Amalias Alla. = Antilla Adg. = Adacidiglossum Amb. = Amblostoma Apr. = Apoda-prorepentia Adcm. = Adacidium Amn. = Amenopsis Aea. = Appletonara Adgm. = Adaglossum Am. = Amesangis Arcp. = Aracampe Adn. = Adamantinia Ams. = Amesara Ara. = Arachnadenia Adm. = Adamara Ame. = Amesiella Arach. = Arachnis Adps. = Adapasia Aml. = Amesilabium Act. = Arachnocentron Adl. = Adelopetalum Ami. = Amitostigma -

Checklist of the Orchidaceae of Panama 165
BOGARÍN et al. — Checklist of the Orchidaceae of Panama 165 CHECKLIST OF THE ORCHIDACEAE OF PANAMA ACIANTHERA Scheidw. Brenesia costaricensis Schltr., Repeeert. Spec. Nov. Regni Veg. Beih. 19: 200. 1923. Acianthera aberrans (Luer) Pupulin & Bogarín, Pleurothallis lateralis L.O.Williams, Ceiba 5: 84. Lankesteriana 8(2): 53. 2008. 1956. Pleurothallis aberrans Luer, Selbyana 2: 382. VOUCHER: not seen, cited by D’Arcy (1987) and Luer 1978. (2003). Aberrantia aberrans (Luer) Luer, Monogr. Syst. Acianthera juxtaposita (Luer) Luer, Monogr. Syst. Bot. Missouri Bot. Gard. 103: 310. 2005. Bot. Missouri Bot. Gard. 95: 253. 2004. VOUCHER: C. Luer & R.L. Dressler 1628 (SEL). Pleurothallis juxtaposita Luer, Lindleyana 6(2): Acianthera butcheri (L.O.Williams) Pridgeon & 103-105. 1991. M.W.Chase, Lindleyana 16: 242. 2001. VOUCHER: C. Luer et al. 10585 (SEL). Pleurothallis butcheri L.O.Williams, Fieldiana, Acianthera lepidota (L.O.Williams) Pridgeon & Bot. 29(6): 346. 1961. M.W.Chase, Lindleyana 16: 244. 2001. Didactylus butcheri (L.O.Williams) Luer, Monogr. Pleurothallis lepidota L.O.Williams, Ann. Missouri Syst. Bot. Missouri Bot. Gard. 95: 257. 2004. Bot. Gard. 27(3): 279., t. 32, f. 81-2. 1940. VOUCHER: H. P. Butcher 651 (F). Acianthera cogniauxiana (Schltr.) Pridgeon & VOUCHER: P.H. Allen 1552 (AMES). M.W.Chase, Lindleyana 16: 243. 2001. Acianthera lojae (Schltr.) Luer, Monogr. Syst. Bot. Pleurothallis cogniauxiana Schltr., Repert. Spec. Missouri Bot. Gard. 95: 254. 2004. Nov. Regni Veg 3(42-43): 246. 1907. Pleurothallis citrophila Luer, Selbyana 3(3-4): Pleurothallis congruens Luer, Selbyana 2(4): 391. 266-267, f. 234. 1977. 1978. Pleurothallis lojae Schltr., Repert. -
OOS Show Classification by Genus 2010
OOS Show Classification by Genus 2010 Preamble Please check the proper class for your entries, and also the spelling of generic names and specific epithets. If you are not absolutely sure that you have identified the proper entry class from the listing in Entry Classes, this document should help to determine the most appropriate class(es). First, check the Quick Reference List (pages 2 and 3): it lists all genera entered in the Ottawa Orchid Society Shows between 2006 and 2009, giving the appropriate class(es) for Orchidophilia 2010. If your genus is not listed there, then proceed to the Comprehensive List (pages 4-30). Any currently accepted genera not listed there are to be entered in Class 115, Orchid species, hybrids and intergenerics not covered elsewhere. As far as practicable, this classification follows the official AOS authorities as specified in the article by Ron McHatton in Awards Quarterly 38(4):287, 2007: these are the World Checklist of Monocotyledons (http://apps.kew.org/wcsp/) for natural genera and the official International Orchid Register (http://apps.rhs.org.uk/horticulturaldatabase/orchidregister/orchidregister.asp) for intergeneric hybrids which is maintained for the International Registration Authority for Orchid Hybrids by the Royal Horticultural Society. The present classification includes synonymy for supplanted intergeneric names and for those natural genera not considered as approved by the World Checklist. It should be noted that intergeneric hybrid nomenclature is in a state of flux at the moment, as the International Registration Authority updates its data to match the delineations proposed in Genera Orchidearum and the recommendations of the International Orchid Commission and the Advisory Panel on Orchid Hybrid Registration. -
Typi Orchidacearum Ab Augusto R. Endresio in Costa Rica Lecti
©Naturhistorisches Museum Wien, download unter www.biologiezentrum.at Ann. Naturhist. Mus. Wien, B 112 265-313 Wien, März 2011 Typi Orchidacearum ab Augusto R. Endresio in Costa Rica lecti F. Pupulin*, C. Ossenbach**, R. Jenny*** & E. Vitek**** Kurzfassung Auguste R. Endres sammelte von Ende 1866 bis 1874 in Costa Rica, für eine kurze Zeit war er auch in Panama. In diesen sieben Jahren widmete er sich insbesonders der Aufsammlung von Orchideen. Nur eine geringe Zahl seiner neuen Funde wurden von ihm selbst publiziert. Elf Arten beschrieb er gemeinsam mit Heinrich Gustav Reichenbach, der weitere 22 Arten aufgrund von Endres' Material beschrieb. Andere Autoren, die mit diesem Material neue Taxa beschrieben, sind Rudolf Schlechter, Fritz Kränzlin und Carlyle A. Luer. Insgesamt wurden 109 Arten und 2 Varietäten auf der Basis von Endres' Sammlungen beschrieben. Hier wird eine kritische Evaluation seiner Orchideentypen in Reichenbach's Sammlungen, die heute im Naturhistorischen Museum Wien deponiert sind, vorgelegt. A bstract Auguste R. Endres botanized in Costa Rica between the end of 1866 and the first months of 1874, spending a short time in Panama. In these seven years he devoted his main attention to the still unrevealed richness of Costa Rican Orchidaceae. The results of his activity have still to be properly evaluated, but his contributions to the botany of Costa Rica are extraordinary in quantity and quality. Notwithstanding his immense labor, only a very small portion of the orchid plants he collected, studied and illustrated were published as new to the science. He co-authored eleven species with Heinrich Gustav Reichenbach, who himself described another 22 species based on his collections. -

Complete Issue
The Vice-Presidency of Research UNIVERSITY OF COSTA RICA is sincerely acknowledged for his support to the printing of this volume ISSN 1409-3871 VOL. 12, No. 1 APRIL 2012 Encylia × nizanburyi (Orchidaceae), un nuevo híbrido natural del istmo de Tehuantepec, México EDUARDO A. PÉREZ-GARCÍA and ERIC HÁGSATER 1 A new species of Campylocentrum (Orchidaceae: Angraecinae) from Colombia MARTA KOLANOWSKA, OSCAR ALEJANDRO PÉREZ ESCOBAR and EDICSON PARRA SÁNCHEZ 9 On the relationship between bryophyte cover and the distribution of Lepanthes spp. BENJAMIN J. CRAIN 13 New species and records of Orchidaceae from Costa Rica. II ADAM P. KARREMANS, DIEGO BOGARÍN, MELANIA FERNÁNDEZ, CHRISTINA M. SMITH and MARIO A. BLANCO 19 New combinations in Odontoglossum (Orchidaceae: Oncidiinae) and a solution to a taxonomic conundrum STIG DALSTRÖM 53 Morfoanatomía en Cranichideae (Orchidaceae) de la Estación Loma Redonda del Parque Nacional “Sierra Nevada”, Mérida, Venezuela BLANCA A. DUGARTE CORREDOR & REBECA LUQUE ARIAS 61 INTERNATIONAL JOURNAL ON ORCHIDOLOGY INTERNATIONAL JOURNAL ON ORCHIDOLOGY Copyright © 2011 Lankester Botanical Garden, University of Costa Rica Effective publication date: April 26, 2012 Layout: Jardín Botánico Lankester. Cover: Acianthera oscitans (Ames) Pridgeon & M.W.Chase. Photograph by F. Pupulin. Printer: Masterlitho Printed copies: 500 Printed in Costa Rica / Impreso en Costa Rica R Lankesteriana / International Journal on Orchidology No. 1 (2001)-- . -- San José, Costa Rica: Editorial Universidad de Costa Rica, 2001-- v. ISSN-1409-3871 1. Botánica - Publicaciones periódicas, 2. Publicaciones periódicas costarricenses Visit the new webpage at www.lankesteriana.org Originally devoted to the publication of articles on general botany, with special attention to epiphytic plants and orchid systematics, ecology, evolution and physiology, along with book reviews and conferen- ces on these subjects, since 2007 LANkeSTERIANA focused exclusively on scientific papers on orchidology.