Extracellular Production of Recombinant Sus Scrofa Trefoil Factor 3 by Brevibacillus Choshinensis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Newsletter UMAP Has Been Promoting Mobility of University Students and Faculties in the Asia-Pacific Region Since 1991
University Mobility in Asia and the Pacific (UMAP) Newsletter UMAP has been promoting mobility of university students and faculties in the Asia-Pacific region since 1991. Starting from 2016, our commemorative 25th 2020/12 anniversary, UMAP would like to periodically inform the general public and our patrons about our activities through this newsletter. This issue of the newsletter Vol. 7 covers the period from October 2019 to December 2020. Contents: 1. New UMAP NS Updates 2. Highlights of the UMAP Board Meeting 2020 3. Report of UMAP-COIL Joint Program 2020 4. Statistical Data 5. UMAP’s Activities 6. Message from UMAP IS New UMAP NS Updates This year, UMAP would like to warmly welcome a couple of new UMAP National Secretariat updates from Mongolia and the United States. • MONGOLIA • UNITED STATES UMAP Mongolia NS was switched to Mongolian Western Washington University is in charge of National Council for Education Accreditation (MNCEA) UMAP U.S.A. NS from January 2020 since Dr. Lee effective as of January 2020. Here’s the primary contact Sternberger, who was a representative of UMAP info as follows. U.S.A. NS and James Madison University, made a career move to Western Washington University. Mrs. Saruul Erdem Here’s the primary contact info as follows. Senior Officer for Foreign Relations Dr. Ryan Larsen [email protected] Director of Study Abroad, Institute for Global +976-9900-1584 Engagement www.accmon.mn [email protected] 360-650-797 Mr. Zorigt Munkhtuya https://www.wwu.edu/ Officer for Foreign Relations [email protected] Dr. Lee Sternberger +976-7010-9391 Executive Director, Institute for Global Engagement [email protected] Ms. -

OC Leadership Development and Education Team Inspire Futre
Kathleen Janson Janson Group 949.654.2512 [email protected] Orange County Leadership Development and Education Team Inspires Future Global Women Leaders in China Suzanne and Dwight Frindt travel to Sias International University to play key role in programs and symposium for the advancement of women San Juan Capistrano, Calif., July 6, 2010 — Few people walk the talk with the unbridled enthusiasm and courage that Suzanne and Dwight Frindt do every day. They are co-founders of 2130 Partners (www.2130Partners.com), a leadership development and education firm training leaders to create focus, alignment and collaboration around a sustainable shared vision. They have just returned from several weeks in China where they participated in The World Academy for the Future of Women, and in the Fourth Annual Women’s Symposium at Sias International University (www.sais.edu.cn/en) in Xinzheng City, in Henan Province, People’s Republic of China. Sias University is the first solely owned American university in Central China. It is designed to develop well-rounded trans-national professionals by combining both Chinese educational philosophies with a Western model of education, providing students with a fresh learning perspective and alternative ways of thinking about business and liberal arts. During this past academic year, the university launched the World Academy for the Future of Women, a rigorous leadership training program conceived by Jerrie Ueberle, founder of the Phoenix, Ariz.-based nonprofit, Global Interactions (www.globalinteractions.org). The program engages women in discovering their purpose and passion in life, and inspires, informs and instructs them in finding their path to success through acquiring skills and confidence to develop as campus, community, national or global leaders. -

International Student Program of Sias University.Pdf Download
y m t a 1 i r g s o 2 r r e P 0 v t i n n e 2 d U - u t S S 0 l A a I 2 n S o i t 0 a n r 2 e t n I From a single semester to multi-year program, students can create their own program alternatively from the core courses Sias University offers or even model their track around their home institution’s needs. Regardless of your current Chinese language level or study objectives in China, we will help you maximize your experience here and prepare you for a global profession. School of International Education SIAS University Tel: 86-371-62608969 Email: [email protected] Letter from SIAS University (SIAS) Dear Students, Greetings from Sias University (SIAS), Zhengzhou, China. Thanks for your interest in studying with us at Sias University. We are excited to connect with you through our International Student Programs. And we would like to invite you to come and learn Chinese language and culture along with many other available courses. Founded in 1998, Sias University was approved by the State Council and licensed by the Henan Provincial Education Commission to carry out higher institutional education. It is an accredited university in China. Sias University offers both degree and non-degree programs for overseas students. Degree programs cover all bachelor degrees offered in the University, while non-degree programs provide overseas students Chinese Language Training Programs in various forms. The aim of Sias University is to develop diligent and innovative professionals to meet the urgent demands of trans-national corporations and enterprises. -
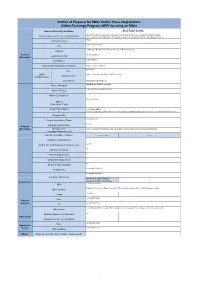
Online Exchange Program (OEP) Focusing on Sdgs
Outline of Proposal for SDGs Online Cross-Registration: Online Exchange Program (OEP) focusing on SDGs Name of University/ Institution Sias University Sias University was founded in 1998 and is the first solely American-owned University in Central Brief Introduction of the University/Institution China. Affiliated with Zhengzhou University, as well as Fort Hays State University of Kansas, USA, it Chinawas developed and designed in response to the most current educational demands. It is the first Country/Territory Zhengzhou Xinzheng City 168 Renmin Rd. Xinzheng, Henan Province. P.R. China 451150 Address General 86-371-62606119 Telephone Number Information Not applicable Fax Number Official University/Institution Website https://www.sias.edu.cn/ Julian Yue Name UMAP Office of International Affairs, Sias University Organization/Office Contact Person Email Address [email protected] International Student Program Name of Program School of International Education Name of Facuty Name of Department Chinese Culture Subject (Code name, if any) Program Description Course Description: & Chinese Culture is a cultural introductive one-semester (2 class hours per week) course for international students who majored in Full English teaching or has required English learning background. Program URL Undergraduate Degree Level and/or Grade English Language of Instruction Program Information Requirements Have a certain level of English expression and be able to participate in discussions in English (Language Proficiency, etc.) Number of Credits to Transfer -

October 2008
GLOBAL INTERACTIONS OCTOBER 2008 BE A MENTOR! GLOBAL MENTORING PROGRAM Contact us SIAS International University Mentoring Project immediately to find This new program is designed to connect a SIAS University out how! senior student with a professional person who will serve as a mentor for the period of November to May. The purpose is to develop a student/advisor relationship that will focus on finding solutions or developing strategies to address current content course work area and/or discover paths to reaching goals beyond the classroom. These could include advanced study, resume development, job search, or sorting through the issues of transition from classroom to workplace. It is a relationship that will build a solid and firm partnership and allow mentors to gain insight to issues important to Chinese university students at SIAS. For more information about SIAS go to WWW.SIAS.EDU.CN Contacts between mentors and mentees will be a minimum of twice per month by e-mail, phone, or SKYPE. We will request a one-page monthly report on the nature of the mentor/mentee exchange so we can use your experience to strengthen and build the program. We will celebrate the success of the program in May at the 10 th Anniversary of SIAS University by convening the mentees and as many mentors as we can to the campus for this celebration. Participation at SIAS is not a requirement for being a mentor. To find out more about this new program contact [email protected] to receive an invitation letter and the Mentor Application. Mentees applications are already arriving through the various clubs and activities at SIAS. -

Annual Report 2014
UNIVERSITY OF GUAM UNIBETSEDÅT GUAHAN Society of Emeritus Professors & Retired Scholars The Society’s Eighteenth Annual Report (2014) Compiled by Cynthia B. Sajnovsky, Professor Emerita of Music, Secretary, SEPRS UOG SEPRS An Overview of the Society’s Eighteenth Annual Report (2014) I want to highlight the vitality that members of the Society of Emeritus Professors and Retired Scholars (SEPRS) have shown in numerous academic, research, and service activities. For many of the UOG community – but not all – their awareness of professors emeriti and retired scholars is “out of sight, out of mind.” Yet this report demonstrates how retired faculty continue to help the University achieve its mission for our community and the region. You can locate specific faculty which are listed alphabetically, and in- time we plan to insert a “search-link” function so you can easily click on a name. SEPRS has 53 current members, of whom half live on Guam (26) and half live off-island across the nation (27). Some of us show the affects of our age, yet3/4ths (38) are active members who participate in the Society’s quarterly meetings, donate to the University, or at least report their activities. In 2014, using IT teleconferencing video technology, we have had five (5) off-island members regularly join and participate in the Society’s quarterly meetings held in Emeritus Hall on-island. So what do these retirees do? Well nearly one-third of the members listed in this report are teaching and mentoring university students. For example at UOG that includes Filomena Contoria, George Kallingal, Lourdes Klitzke, and Milagros Moguel, and off-island take a look at Joyce McCauley. -

GAP YEAR in CHINA with the WORLD ACADEMY for the FUTURE of WOMEN
GAP YEAR IN CHINA with the WORLD ACADEMY FOR THE FUTURE OF WOMEN At Sias International University through collaboration with Global Interactions “Discovering your passion and purpose enables you to find your voice, take action and create a life you never dreamed possible.” Jerrie Ueberle “探索激情和目标,能让你发现自己的心声,行动起来,创造一个梦想不到的人生。” ——杰瑞·尤伯利 www.wafw.org 2 WHAT IS A WAFW GAP YEAR? A Gap Year is an opportunity for high school graduates and/or university students to experience another culture and to widen their perspective while advancing their aca- demic careers. The World Academy for the Future of Women (WAFW) partnered with SIAS International University to make this experience even richer for Gap Year students. WAFW enables student members to find their passion, purpose and path to success. Members practice leadership in action and learn what it takes to become a global citizen and leader. Students will gain a broader perspective for what is possible in choosing their major and potential careers to envision their future and the path to get there. Why CHINA? One in four people on the planet are Chinese. SIAS International University is an ‘East meets West’ campus where students speak English and are eager to learn more about the west. The exchange with American and western students is ro- bust with real time learning and relationship building in the fastest growing economy in the world. Immersion in Mandarin accelerates and deepens learning and the ability to connect authentically with this ancient and dynamic culture. LEARN MORE: World Academy -

A Report on Community Colleges and Science and Civic Engagement in Asia: a Yearlong and Continuing Journey
POINT OF VIEW A Report on Community Colleges and Science and Civic Engagement in Asia: A Yearlong and Continuing Journey Robert W. Franco University of Hawai’i Throughout 2013 I had the opportunity to travel to East and civilization nearly 5,000 years ago. Today, more than 100 South Asia, to explore connections between the work of com- million people live in Henan, which is two-thirds the size of munity colleges and civically engaged science. What follows Arizona. Although the Yellow River does not flow through is a general summary of what was learned through a com- Henan Province as it once did, the river skirts the boundaries bination of ethnographic observation and ongoing scholarly of the Sias campus. engagement with Sias University in China, the Asia Pacific Dr. Paul Elsner, who for 22 years served as Chancellor Higher Education Research Program (APHERP) at the of the 10-campus Maricopa Community College System, in- East-West Center in Hawai’i, and the University of Mumbai vited me to make a presentation at the Sias University In- in India. The report will conclude by suggesting how these ternational Water Conference, May 22–25, 2013. Dr. Elsner international interactions relate to a new Teagle Foundation- knew that Kapi’olani Community College (KCC) and the funded project at Kapi’olani Community College and the University of Hawai’i at Manoa (UHM) had developed and Community College National Center for Community En- sustained a strong service learning and civic engagement pro- gagement (CCNCCE). gram called Malama i na Ahupua’a (to care for the ahupua’a), which engages students and faculty in restoring ancient Ha- waiian watersheds throughout the island of O’ahu. -

Innovating for International Enrollment Success
Wednesday, February 19 | 8:00 am to 9:00 am | Room: Delaware B Rethinking Internationalization with Innovative Partnerships: What Works, What Doesn’t? • Rahul Choudaha, Principal Researcher, DrEducation • Gbemi Disu, Chief Business Officer, George Mason University Korea • Cindy Elliott, Assistant Vice President for Global Partnerships, Fort Hays State University • Rahim Karim, Associate VP Partnerships, Pathways & Internationalization, Centennial College • Tayyeb Shah, Deputy Vice-Chancellor (Global Partnerships), The University of Western Australia • Brent White, Vice Provost for Global Affairs, University of Arizona Innovative partnerships overcome constraints to deliver results… Partnership is not a posture but a process-a Managing innovation will increasingly become a continuous process that grows stronger each challenge to management, and especially to top year as we devote ourselves to common tasks. management, and a touchstone of its competence. - JF Kennedy - Peter Drucker FORT HAYS STATE Today, 4,500 students at two partner universities: UNIVERSITY • Sias University (Henan) Established in 1902, FHSU is located in Hays, Kansas. • Shenyang Normal University (Liaoning) 1999 2019 On campus 5,600 4,485 6 different degrees: BBA; BS; and BA Online 600 6,961 Graduated over 10,000 Chinese students. Mainland China 0 4,453 * Total 6,200 15,899 All fully employed or studying post- graduate education in US, China or other Making FHSU the third largest public university in countries. Kansas, offering 200+ degrees. FHSU’s Model for 4+0 Cross Border In 2000, FHSU was the first American university to be Education: FHSU doesn’t’ own a campus; approved by MOE to offer UG dual degrees. Started has partnerships; students not required to with 40 students. -

Visa, Travel, and Quarantine Expectations and Recommendations 2021-2022
Visa, Travel, and Quarantine Expectations and Recommendations 2021-2022 Table of Contents Validity of Documents ............................................................................................................................................................ 1 Travel Expectations and Recommendations – China 2021 ................................................................................................... 1 Pre-Departure ..................................................................................................................................................................... 1 All travelers must complete: ........................................................................................................................................... 1 A green health [QR] code will be issued before departure, once you test negative. ..................................................... 1 Packing and Preparation Suggestions ................................................................................................................................ 2 Packing Essentials ........................................................................................................................................................... 2 Packing Recommendations – In checked luggage .......................................................................................................... 2 Suggested food items ..................................................................................................................................................... -

SIAS International University , China 27-28 June, 2016
PHOTOGRAPHY AND FILMING Photographs and video footage will be taken throughout the Festival for use in promotional material, if you would not like to be featured, speak to the photographer directly. ENQUIRIES Key contacts for the Global Festival of Learning at Sias Dr Lucy Lu Associate Dean (Global Engagement), Faculty of Management Email: [email protected] Mobile: 0044 7710763519 (UK); 0086 13120358289 (China) Mr William Lu Director - International Cooperation & Exchange Department Sias International University, 168 Renmin Rd. Xinzheng City Zhengzhou, Henan Province, P.R. China 451150 Email: [email protected] Office Phone & Fax: +86-371-69972860 Mobile: +86-185-3002-9955 Mr Gavin Chen International Cooperation & Exchange Department of Sias International University 168 East, Renmin Road, Xinzheng City, Zhengzhou, Henan, China (451150) Mobile: 86-18638643423 Tel: 86-371-62600541 SIAS International University , China 27-28 June, 2016 www.bournemouth.ac.uk/fol 7805-06/16 CONTENTS Welcome 3 WELCOME Monday 27 June 5 Parallel session presentations 6 Tuesday 28 June 10 Staff profiles 12 How to find us 19 Bookings and enquiries 20 We are delighted to welcome you to our Festival of Learning China event, which presents an exciting opportunity to showcase the About the Festival of Learning expertise of both our institutions and regions. Over the course China at SIAS International University of the next couple of days, we hope you will enjoy and engage with the diverse and interesting range of interactive sessions On the following pages, you will find details of the events taking place at SIAS International University from the 27th to the 28th June as part of Bournemouth that will be co-delivered by staff and students of Bournemouth University’s Festival of Learning, China. -
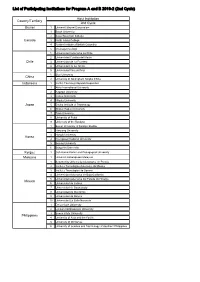
List of Participating Institutions for Program a and B 2019-2 (2Nd Cycle)
List of Participating Institutions for Program A and B 2019-2 (2nd Cycle) Host Institution Country/Territory 2nd Cycle Brunei 1 Universiti Brunei Darussalam 1 Brock University 2 Coast Mountain College Canada 3 North Island College 4 Justice Institute of British Columbia 5 Okanagan College 1 Universidad Autonoma de Chile 2 Universidad Catolica del Maule Chile 3 Universidad de La Frontera 4 Universidad de los Andes 5 Universidad Vina del Mar 1 Sias University China 2 University of Nottingham Ningbo China Indonesia 1 Institut Teknologi Sepuluh Nopember 1 Akita International University 2 Kagawa University 3 Kansai University 4 Niigata University Japan 5 Osaka Institute of Technology 6 Shokei Gakuin University 7 Toyo University 8 University of Fukui 9 University of the Ryukyus 1 Busan University of Foreign Studies 2 Hanyang University 3 Hongik University Korea 4 Kyungpook National University 5 Sejong University 6 Sungshin University Kyrgyz 1 Osh Humanitarian and Pedagogical University Malaysia 1 Universiti Kebangsaan Malaysia 1 Benemerita Universidad Autonoma de Puebla 2 Instituto Tecnologico Autonomo de Mexico 3 Instituto Tecnologico de Sonora 4 Universidad Autonoma de Baja California 5 Universidad Autonoma del Estado de Hidalgo Mexico 6 Universidad de Colima 7 Universidad de Guanajuato 8 Universidad de Monterrey 9 Universidad de Sonora 10 Universidad La Salle Noroeste 1 De La Salle University 2 Lyceum Northwestern University 3 Samar State University Philippines 4 University of Asia and the Pacific 5 University of Mindanao 6 University of