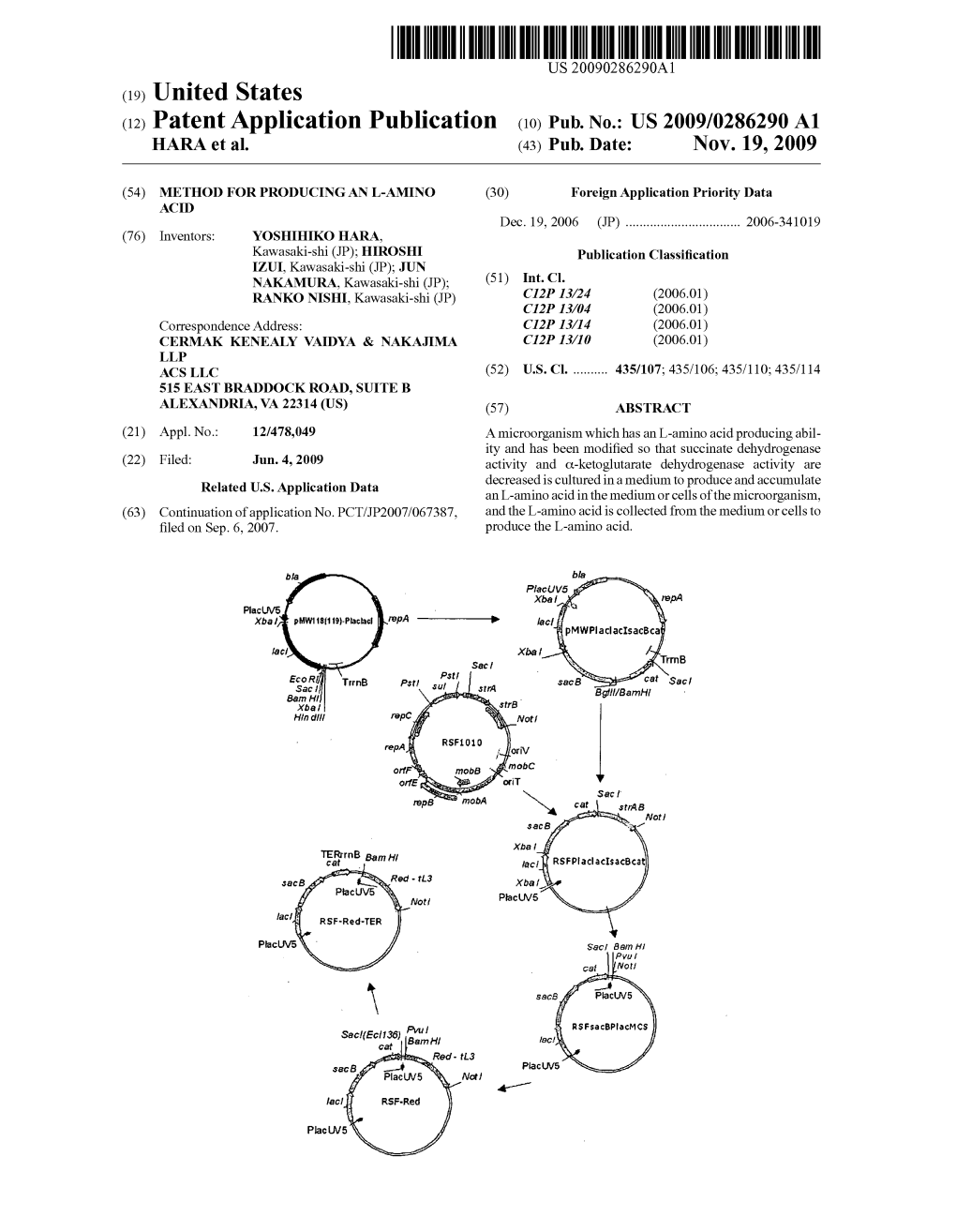
(12) Patent Application Publication (10) Pub. No.: US 2009/0286290 A1 HARA Et Al
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Synthesis and Antifungal Properties of Compounds Which Target the Α-Aminoadipate Pathway ORIGINAL ARTICLES
ORIGINAL ARTICLES Department of Chemistry 1 and Department of Biology2, University of Saskatchewan, Canada Synthesis and antifungal properties of compounds which target the a-aminoadipate pathway D. R. J. Palmer,1 H. Balogh,1 G. Ma,1 X. Zhou,1 M. Marko,1 S. G. W. Kaminskyj 2 Received June 20, 2003, accepted July 22, 2003 Susan G. W. Kaminskyj, Department of Biology, University of Saskatchewan, 112 Science Place, Saskatoon, Saskatchewan Canada S7N 5E2. [email protected] Pharmazie 59: 93–98 (2004) Fungi synthesize lysine via the a-aminoadipate pathway, which is not found in plants or animals. This pathway has been proposed as a target for antifungal agents, but until now no reports have appeared to test this proposal. Hampering studies on the susceptibility of filamentous fungi such as those of the clinically important genus Aspergillus is the fact that growth quantitation is notoriously difficult. We have used the recently-reported XTT-based method of biomass quantitation to measure the suscept- ibility of Aspergillus nidulans strain A28 to growth suppression by novel compounds designed to target early steps in the a-aminoadipate lysine biosynthesis pathway, specifically those steps involving (R)- homocitrate and (2R,3S)-homoisocitrate. Three compounds show moderate inhibition of fungal growth, which can be partially restored by the presence of lysine in the growth medium. 1. Introduction All higher fungi synthesize lysine via the a-aminoadipate pathway (Zabriskie and Jackson 2000), which is not found Invasive fungal infections are a severe threat to human in plants or animals. This pathway could therefore serve health: untreated, mortality rates from systemic fungal infections typically exceed 98%. -

12) United States Patent (10
US007635572B2 (12) UnitedO States Patent (10) Patent No.: US 7,635,572 B2 Zhou et al. (45) Date of Patent: Dec. 22, 2009 (54) METHODS FOR CONDUCTING ASSAYS FOR 5,506,121 A 4/1996 Skerra et al. ENZYME ACTIVITY ON PROTEIN 5,510,270 A 4/1996 Fodor et al. MICROARRAYS 5,512,492 A 4/1996 Herron et al. 5,516,635 A 5/1996 Ekins et al. (75) Inventors: Fang X. Zhou, New Haven, CT (US); 5,532,128 A 7/1996 Eggers Barry Schweitzer, Cheshire, CT (US) 5,538,897 A 7/1996 Yates, III et al. s s 5,541,070 A 7/1996 Kauvar (73) Assignee: Life Technologies Corporation, .. S.E. al Carlsbad, CA (US) 5,585,069 A 12/1996 Zanzucchi et al. 5,585,639 A 12/1996 Dorsel et al. (*) Notice: Subject to any disclaimer, the term of this 5,593,838 A 1/1997 Zanzucchi et al. patent is extended or adjusted under 35 5,605,662 A 2f1997 Heller et al. U.S.C. 154(b) by 0 days. 5,620,850 A 4/1997 Bamdad et al. 5,624,711 A 4/1997 Sundberg et al. (21) Appl. No.: 10/865,431 5,627,369 A 5/1997 Vestal et al. 5,629,213 A 5/1997 Kornguth et al. (22) Filed: Jun. 9, 2004 (Continued) (65) Prior Publication Data FOREIGN PATENT DOCUMENTS US 2005/O118665 A1 Jun. 2, 2005 EP 596421 10, 1993 EP 0619321 12/1994 (51) Int. Cl. EP O664452 7, 1995 CI2O 1/50 (2006.01) EP O818467 1, 1998 (52) U.S. -

A Prokaryotic Gene Cluster Involved in Synthesis of Lysine Through the Amino Adipate Pathway: a Key to the Evolution of Amino Acid Biosynthesis
Downloaded from genome.cshlp.org on September 28, 2021 - Published by Cold Spring Harbor Laboratory Press Research A Prokaryotic Gene Cluster Involved in Synthesis of Lysine through the Amino Adipate Pathway: A Key to the Evolution of Amino Acid Biosynthesis Hiromi Nishida,1 Makoto Nishiyama,2,4 Nobuyuki Kobashi,2 Takehide Kosuge,3 Takayuki Hoshino,3 and Hisakazu Yamane2 1Institute of Molecular and Cellular Biosciences, The University of Tokyo, Tokyo 113-0032, Japan; 2Biotechnology Research Center, The University of Tokyo, Tokyo 113-8657, Japan; 3Institute of Applied Biochemistry, University of Tsukuba, Tsukuba, Ibaraki 305-8572, Japan In previous studies we determined the nucleotide sequence of the gene cluster containing lys20, hacA (lys4A), hacB (lys4B), orfE, orfF, rimK, argC, and argB of Thermus thermophilus, an extremely thermophilic bacterium. In this study, we characterized the role of each gene in the cluster by gene disruption and examined auxotrophy in the disruptants. All disruptants except for the orfE disruption showed a lysine auxotrophic phenotype. This was surprising because this cluster consists of genes coding for unrelated proteins based on their names, which had been tentatively designated by homology analysis. Although the newly found pathway contains ␣-aminoadipic acid as a lysine biosynthetic intermediate, this pathway is not the same as the eukaryotic one. When each of the gene products was phylogenetically analyzed, we found that genes evolutionarily-related to the lysine biosynthetic genes in T. thermophilus were all present in a hyperthermophilic and anaerobic archaeon, Pyrococcus horikoshii, and formed a gene cluster in a manner similar to that in T. thermophilus. Furthermore, this gene cluster was analogous in part to the present leucine and arginine biosyntheses pathways. -

Metabolic Engineering of Corynebacterium Glutamicum for Production of the Adipate
Metabolic engineering of Corynebacterium glutamicum for production of the adipate precursor 2-oxoadipate A dissertation submitted to the Mathematisch-Naturwissenschaftliche Fakultät of the Heinrich-Heine-Universität Düsseldorf presented by Markus Sebastian Spelberg born in Krefeld October 2013 The thesis in hand has been performed at the Institute of Bio- and Geosciences, IBG-1: Biotechnology, Forschungszentrum Jülich GmbH, from September 2009 until October 2013 under the supervision of Prof. Dr. Michael Bott. Printed with the permission of the Mathematisch-Naturwissenschaftliche Fakultät of the Heinrich-Heine-Universität Düsseldorf Examiner: Prof. Dr. Michael Bott Institute of Bio- and Geosciences 1, IBG-1: Biotechnology Forschungszentrum Jülich GmbH Coexaminer: PD Dr. Ulrich Schulte Department of Biochemistry I Heinrich-Heine-Universität Düsseldorf th Date of the oral examination: 5 December 2013 Content CONTENT 1 Summary ............................................................................................................................ 1 1.1 Summary ...................................................................................................................... 1 1.2 Zusammenfassung ....................................................................................................... 3 2 Introduction ........................................................................................................................ 5 2.1 Significance and application of adipate ...................................................................... -

Figure1 Gabriel
E u k a E Unikonts Ch A r x y r c ota chaeplastida r a omal va V t iridiplantae F e M un v s eola eta Di g k i z a t oa r es y a He R Hap C E Api Ciliophora Al P Neocallimastigo Amoeb F Basidio M Choan A St R A M Pla Nema Cnidaria Pla P Chlo Glau Cho ornicata uglen arabasalia o h hodop r s r ollusca ic r v yp th c r i ep t t c eolata za i c o e r yhelminthe f r oz t osporidia r omplexa e c r t r m data op opoda t op r okon op op r t op i oa o a oda a y o m o h z c h h h z h z h oa yta ota y oa yta yta yta yta oa yta c t ota op h s yta m y c ota ATP phosphoribosyltransferase H1 phosphoribosyl-ATP pyrophosphohydrolase H2 Phosphoribosyl-AMP cyclohydrolase H3 5-proFAR isomerase H4 H Glutamine amidotransferase H5 Imidazoleglycerol-phosphate dehydratase H6 Histidinol-phosphate aminotransferase H7 Histidinol phosphatase H8 Histidinol dehydrogenase H9 3-deoxy-7-phosphoheptulonate synthase FW1 3-dehydroquinate synthase FW2 3-dehydroquinate dehydratase FW3 Shikimate 5-dehydrogenase FW4 Shikimate kinase FW5 F 3-phosphoshikimate 1-carboxyvinyltransferase FW6 Chorismate synthase FW7 Chorismate mutase F8 Prephenate dehydratase F9 4 distinct aminotransferases F10 3-deoxy-7-phosphoheptulonate synthase FW1 3-dehydroquinate synthase FW2 3-dehydroquinate dehydratase FW3 Shikimate 5-dehydrogenase FW4 Shikimate kinase FW5 3-phosphoshikimate 1-carboxyvinyltransferase FW6 W Chorismate synthase FW7 Anthranilate synthase W8 Anthranilate phosphoribosyltransferase W9 N(5`-phosphoribosyl) anthranilate isomerase W10 Indole-3-glycerol phosphate synthase W11 Tryptophan -

POLSKIE TOWARZYSTWO BIOCHEMICZNE Postępy Biochemii
POLSKIE TOWARZYSTWO BIOCHEMICZNE Postępy Biochemii http://rcin.org.pl WSKAZÓWKI DLA AUTORÓW Kwartalnik „Postępy Biochemii” publikuje artykuły monograficzne omawiające wąskie tematy, oraz artykuły przeglądowe referujące szersze zagadnienia z biochemii i nauk pokrewnych. Artykuły pierwszego typu winny w sposób syntetyczny omawiać wybrany temat na podstawie możliwie pełnego piśmiennictwa z kilku ostatnich lat, a artykuły drugiego typu na podstawie piśmiennictwa z ostatnich dwu lat. Objętość takich artykułów nie powinna przekraczać 25 stron maszynopisu (nie licząc ilustracji i piśmiennictwa). Kwartalnik publikuje także artykuły typu minireviews, do 10 stron maszynopisu, z dziedziny zainteresowań autora, opracowane na podstawie najnow szego piśmiennictwa, wystarczającego dla zilustrowania problemu. Ponadto kwartalnik publikuje krótkie noty, do 5 stron maszynopisu, informujące o nowych, interesujących osiągnięciach biochemii i nauk pokrewnych, oraz noty przybliżające historię badań w zakresie różnych dziedzin biochemii. Przekazanie artykułu do Redakcji jest równoznaczne z oświadczeniem, że nadesłana praca nie była i nie będzie publikowana w innym czasopiśmie, jeżeli zostanie ogłoszona w „Postępach Biochemii”. Autorzy artykułu odpowiadają za prawidłowość i ścisłość podanych informacji. Autorów obowiązuje korekta autorska. Koszty zmian tekstu w korekcie (poza poprawieniem błędów drukarskich) ponoszą autorzy. Artykuły honoruje się według obowiązujących stawek. Autorzy otrzymują bezpłatnie 25 odbitek swego artykułu; zamówienia na dodatkowe odbitki (płatne) należy zgłosić pisemnie odsyłając pracę po korekcie autorskiej. Redakcja prosi autorów o przestrzeganie następujących wskazówek: Forma maszynopisu: maszynopis pracy i wszelkie załączniki należy nadsyłać w dwu egzem plarzach. Maszynopis powinien być napisany jednostronnie, z podwójną interlinią, z marginesem ok. 4 cm po lewej i ok. 1 cm po prawej stronie; nie może zawierać więcej niż 60 znaków w jednym wierszu nie więcej niż 30 wierszy na stronie zgodnie z Normą Polską. -

Physiological Models of Geobacter Sulfurreducens and Desulfobacter Postgatei to Understand Uranium Remediation in Subsurface Systems
University of Massachusetts Amherst ScholarWorks@UMass Amherst Doctoral Dissertations Dissertations and Theses November 2014 PHYSIOLOGICAL MODELS OF GEOBACTER SULFURREDUCENS AND DESULFOBACTER POSTGATEI TO UNDERSTAND URANIUM REMEDIATION IN SUBSURFACE SYSTEMS Roberto Orellana University of Massachusetts Amherst Follow this and additional works at: https://scholarworks.umass.edu/dissertations_2 Part of the Environmental Microbiology and Microbial Ecology Commons, and the Microbial Physiology Commons Recommended Citation Orellana, Roberto, "PHYSIOLOGICAL MODELS OF GEOBACTER SULFURREDUCENS AND DESULFOBACTER POSTGATEI TO UNDERSTAND URANIUM REMEDIATION IN SUBSURFACE SYSTEMS" (2014). Doctoral Dissertations. 278. https://doi.org/10.7275/5822237.0 https://scholarworks.umass.edu/dissertations_2/278 This Open Access Dissertation is brought to you for free and open access by the Dissertations and Theses at ScholarWorks@UMass Amherst. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of ScholarWorks@UMass Amherst. For more information, please contact [email protected]. PHYSIOLOGICAL MODELS OF GEOBACTER SULFURREDUCENS AND DESULFOBACTER POSTGATEI TO UNDERSTAND URANIUM REMEDIATION IN SUBSURFACE SYSTEMS A Dissertation Presented by ROBERTO ORELLANA ROMAN Submitted to the Graduate School of the University of Massachusetts Amherst in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY September 2014 Microbiology Department © Copyright by Roberto Orellana Roman 2014 All Rights -

(12) Patent Application Publication (10) Pub. No.: US 2012/0266329 A1 Mathur Et Al
US 2012026.6329A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2012/0266329 A1 Mathur et al. (43) Pub. Date: Oct. 18, 2012 (54) NUCLEICACIDS AND PROTEINS AND CI2N 9/10 (2006.01) METHODS FOR MAKING AND USING THEMI CI2N 9/24 (2006.01) CI2N 9/02 (2006.01) (75) Inventors: Eric J. Mathur, Carlsbad, CA CI2N 9/06 (2006.01) (US); Cathy Chang, San Marcos, CI2P 2L/02 (2006.01) CA (US) CI2O I/04 (2006.01) CI2N 9/96 (2006.01) (73) Assignee: BP Corporation North America CI2N 5/82 (2006.01) Inc., Houston, TX (US) CI2N 15/53 (2006.01) CI2N IS/54 (2006.01) CI2N 15/57 2006.O1 (22) Filed: Feb. 20, 2012 CI2N IS/60 308: Related U.S. Application Data EN f :08: (62) Division of application No. 1 1/817,403, filed on May AOIH 5/00 (2006.01) 7, 2008, now Pat. No. 8,119,385, filed as application AOIH 5/10 (2006.01) No. PCT/US2006/007642 on Mar. 3, 2006. C07K I4/00 (2006.01) CI2N IS/II (2006.01) (60) Provisional application No. 60/658,984, filed on Mar. AOIH I/06 (2006.01) 4, 2005. CI2N 15/63 (2006.01) Publication Classification (52) U.S. Cl. ................... 800/293; 435/320.1; 435/252.3: 435/325; 435/254.11: 435/254.2:435/348; (51) Int. Cl. 435/419; 435/195; 435/196; 435/198: 435/233; CI2N 15/52 (2006.01) 435/201:435/232; 435/208; 435/227; 435/193; CI2N 15/85 (2006.01) 435/200; 435/189: 435/191: 435/69.1; 435/34; CI2N 5/86 (2006.01) 435/188:536/23.2; 435/468; 800/298; 800/320; CI2N 15/867 (2006.01) 800/317.2: 800/317.4: 800/320.3: 800/306; CI2N 5/864 (2006.01) 800/312 800/320.2: 800/317.3; 800/322; CI2N 5/8 (2006.01) 800/320.1; 530/350, 536/23.1: 800/278; 800/294 CI2N I/2 (2006.01) CI2N 5/10 (2006.01) (57) ABSTRACT CI2N L/15 (2006.01) CI2N I/19 (2006.01) The invention provides polypeptides, including enzymes, CI2N 9/14 (2006.01) structural proteins and binding proteins, polynucleotides CI2N 9/16 (2006.01) encoding these polypeptides, and methods of making and CI2N 9/20 (2006.01) using these polynucleotides and polypeptides. -

All Enzymes in BRENDA™ the Comprehensive Enzyme Information System
All enzymes in BRENDA™ The Comprehensive Enzyme Information System http://www.brenda-enzymes.org/index.php4?page=information/all_enzymes.php4 1.1.1.1 alcohol dehydrogenase 1.1.1.B1 D-arabitol-phosphate dehydrogenase 1.1.1.2 alcohol dehydrogenase (NADP+) 1.1.1.B3 (S)-specific secondary alcohol dehydrogenase 1.1.1.3 homoserine dehydrogenase 1.1.1.B4 (R)-specific secondary alcohol dehydrogenase 1.1.1.4 (R,R)-butanediol dehydrogenase 1.1.1.5 acetoin dehydrogenase 1.1.1.B5 NADP-retinol dehydrogenase 1.1.1.6 glycerol dehydrogenase 1.1.1.7 propanediol-phosphate dehydrogenase 1.1.1.8 glycerol-3-phosphate dehydrogenase (NAD+) 1.1.1.9 D-xylulose reductase 1.1.1.10 L-xylulose reductase 1.1.1.11 D-arabinitol 4-dehydrogenase 1.1.1.12 L-arabinitol 4-dehydrogenase 1.1.1.13 L-arabinitol 2-dehydrogenase 1.1.1.14 L-iditol 2-dehydrogenase 1.1.1.15 D-iditol 2-dehydrogenase 1.1.1.16 galactitol 2-dehydrogenase 1.1.1.17 mannitol-1-phosphate 5-dehydrogenase 1.1.1.18 inositol 2-dehydrogenase 1.1.1.19 glucuronate reductase 1.1.1.20 glucuronolactone reductase 1.1.1.21 aldehyde reductase 1.1.1.22 UDP-glucose 6-dehydrogenase 1.1.1.23 histidinol dehydrogenase 1.1.1.24 quinate dehydrogenase 1.1.1.25 shikimate dehydrogenase 1.1.1.26 glyoxylate reductase 1.1.1.27 L-lactate dehydrogenase 1.1.1.28 D-lactate dehydrogenase 1.1.1.29 glycerate dehydrogenase 1.1.1.30 3-hydroxybutyrate dehydrogenase 1.1.1.31 3-hydroxyisobutyrate dehydrogenase 1.1.1.32 mevaldate reductase 1.1.1.33 mevaldate reductase (NADPH) 1.1.1.34 hydroxymethylglutaryl-CoA reductase (NADPH) 1.1.1.35 3-hydroxyacyl-CoA -

KO Id Functional Hierarchy 1 Functional Hierarchy 2 Module Id Module Description Name Definition Life Style K00611 Amino Acid Me
KO_id Functional hierarchy 1 Functional hierarchy 2 Module_id Module description Name Definition Life_style K00611 Amino acid metabolism Arginine and proline metabolism M00029 Urea cycle [PATH:map00220 map01230 map01100] OTC, argF, argI ornithine carbamoyltransferase [EC:2.1.3.3] FL-ls K00611 Amino acid metabolism Arginine and proline metabolism M00844 Arginine biosynthesis, ornithine => arginine [PATH:map00220OTC, map01230argF, argI map01100]ornithine carbamoyltransferase [EC:2.1.3.3] FL-ls K00286 Amino acid metabolism Arginine and proline metabolism M00015 Proline biosynthesis, glutamate => proline [PATH:map00330proC map01230 map01100]pyrroline-5-carboxylate reductase [EC:1.5.1.2] PA-ls K00818 Amino acid metabolism Arginine and proline metabolism M00028 Ornithine biosynthesis, glutamate => ornithine [PATH:map00220E2.6.1.11, map01210 argD map01230acetylornithine map01100] aminotransferase [EC:2.6.1.11] PA-ls K00619 Amino acid metabolism Arginine and proline metabolism M00028 Ornithine biosynthesis, glutamate => ornithine [PATH:map00220argA map01210 map01230amino-acid map01100] N-acetyltransferase [EC:2.3.1.1] PA-ls K00620 Amino acid metabolism Arginine and proline metabolism M00028 Ornithine biosynthesis, glutamate => ornithine [PATH:map00220argJ map01210 map01230glutamate map01100] N-acetyltransferase / amino-acid N-acetyltransferasePA-ls [EC:2.3.1.35 2.3.1.1] K00930 Amino acid metabolism Arginine and proline metabolism M00028 Ornithine biosynthesis, glutamate => ornithine [PATH:map00220argB map01210 map01230acetylglutamate map01100] -

(12) Patent Application Publication (10) Pub. No.: US 2015/0240226A1 Mathur Et Al
US 20150240226A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2015/0240226A1 Mathur et al. (43) Pub. Date: Aug. 27, 2015 (54) NUCLEICACIDS AND PROTEINS AND CI2N 9/16 (2006.01) METHODS FOR MAKING AND USING THEMI CI2N 9/02 (2006.01) CI2N 9/78 (2006.01) (71) Applicant: BP Corporation North America Inc., CI2N 9/12 (2006.01) Naperville, IL (US) CI2N 9/24 (2006.01) CI2O 1/02 (2006.01) (72) Inventors: Eric J. Mathur, San Diego, CA (US); CI2N 9/42 (2006.01) Cathy Chang, San Marcos, CA (US) (52) U.S. Cl. CPC. CI2N 9/88 (2013.01); C12O 1/02 (2013.01); (21) Appl. No.: 14/630,006 CI2O I/04 (2013.01): CI2N 9/80 (2013.01); CI2N 9/241.1 (2013.01); C12N 9/0065 (22) Filed: Feb. 24, 2015 (2013.01); C12N 9/2437 (2013.01); C12N 9/14 Related U.S. Application Data (2013.01); C12N 9/16 (2013.01); C12N 9/0061 (2013.01); C12N 9/78 (2013.01); C12N 9/0071 (62) Division of application No. 13/400,365, filed on Feb. (2013.01); C12N 9/1241 (2013.01): CI2N 20, 2012, now Pat. No. 8,962,800, which is a division 9/2482 (2013.01); C07K 2/00 (2013.01); C12Y of application No. 1 1/817,403, filed on May 7, 2008, 305/01004 (2013.01); C12Y 1 1 1/01016 now Pat. No. 8,119,385, filed as application No. PCT/ (2013.01); C12Y302/01004 (2013.01); C12Y US2006/007642 on Mar. 3, 2006. -
A Dissertation Entitled Development of Selective Inhibitors Against
A Dissertation entitled Development of Selective Inhibitors against Enzymes Involved in the Aspartate Biosynthetic Pathway for Antifungal Drug Development By Gopal P. Dahal Submitted to the Graduate Faculty as partial fulfillment of the requirements for the Doctor of Philosophy Degree in Chemistry ________________________________________ Dr. Ronald E. Viola, Committee Chair ________________________________________ Dr. Donald Ronning, Committee Member ________________________________________ Dr. Jianglong Zhu, Committee Member ________________________________________ Dr. Viranga Tillekeratne, Committee Member ________________________________________ Dr. Amanda C. Bryant-Friedrich, Dean College of Graduate Studies The University of Toledo August 2018 © Copyright by Gopal P. Dahal, 2018 This document is copyrighted material. Under copyright law, no parts of this document may be reproduced without the expressed permission of the author. An Abstract of Development of Selective Inhibitors against Enzymes Involved in the Aspartate Biosynthetic Pathway for Antifungal Drug Development by Gopal P. Dahal Submitted to the Graduate Faculty as partial fulfillment of the requirements for the Doctor of Philosophy Degree in Chemistry The University of Toledo August 2018 Aspartate semialdehyde dehydrogenase (ASADH) functions at a critical junction in the aspartate biosynthetic pathway and represents a validated target for antimicrobial drug design. This enzyme catalyzes the NADPH-dependent reductive dephosphorylation of β-aspartyl phosphate to produce the key intermediate aspartate semialdehyde. Production of this intermediate represents the first committed step for the biosynthesis of several essential amino acids in fungi and in bacteria. The absence of this enzyme in humans and other mammals will allow the selective targeting of any ASADH inhibitors against pathogenic microorganisms. We have accumulated significant structural and mechanistic information about the bacterial ASADHs, but have only limited knowledge of their fungal counterparts.