Through-Process Modeling of Aluminum Alloys for Cold Spray: EXPERIMENTAL CHARACTERIZATION and VERIFICATION of MODELS
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Paclitaxel-Loaded Glycyrrhizic Acid Micelles
Molecules 2015, 20, 4337-4356; doi:10.3390/molecules20034337 OPEN ACCESS molecules ISSN 1420-3049 www.mdpi.com/journal/molecules Article Bioavailability Enhancement of Paclitaxel via a Novel Oral Drug Delivery System: Paclitaxel-Loaded Glycyrrhizic Acid Micelles Fu-Heng Yang †, Qing Zhang †, Qian-Ying Liang, Sheng-Qi Wang, Bo-Xin Zhao, Ya-Tian Wang, Yun Cai and Guo-Feng Li * Department of Pharmacy, Nanfang Hospital, Southern Medical University, Guangzhou 510515, China; E-Mails: [email protected] (F.-H.Y.); [email protected] (Q.Z.); [email protected] (Q.-Y.L.); [email protected] (S.-Q.W.); [email protected] (B.-X.Z.); [email protected] (Y.-T.W.); [email protected] (Y.C.) † These authors contributed equally to this work. * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +86-20-6278-7236; Fax: +86-20-6278-7724. Academic Editor: Derek J. McPhee Received: 7 November 2014 / Accepted: 25 February 2015 / Published: 6 March 2015 Abstract: Paclitaxel (PTX, taxol), a classical antitumor drug against a wide range of tumors, shows poor oral bioavailability. In order to improve the oral bioavailability of PTX, glycyrrhizic acid (GA) was used as the carrier in this study. This was the first report on the preparation, characterization and the pharmacokinetic study in rats of PTX-loaded GA micelles The PTX-loaded micelles, prepared with ultrasonic dispersion method, displayed small particle sizes and spherical shapes. Differential scanning calorimeter (DSC) thermograms indicated that PTX was entrapped in the GA micelles and existed as an amorphous state. The encapsulation efficiency was about 90%, and the drug loading rate could reach up to 7.90%. -

Types of Solutions
Chemistry 51 Chapter 8 TYPES OF SOLUTIONS A solution is a homogeneous mixture of two substances: a solute and a solvent. Solute: substance being dissolved; present in lesser amount. Solvent: substance doing the dissolving; present in larger amount. Solutes and solvents may be of any form of matter: solid, liquid or gas. Some Examples of Solutions Type Example Solute Solvent Gas in gas Air Oxygen (gas) Nitrogen (gas) Gas in liquid Soda water CO2 (gas) Water (liquid) Liquid in liquid Vinegar Acetic acid (liquid) Water (liquid) Solid in liquid Seawater Salt (solid) Water (liquid) Liquid in solid Dental amalgam Mercury (liquid) Silver (solid) Solid in solid Brass Zinc (solid) Copper (solid) Solutions form between solute and solvent molecules because of similarities between them. (Like dissolves Like) Ionic solids dissolve in water because the charged ions (polar) are attracted to the polar water molecules. Non-polar molecules such as oil and grease dissolve in non-polar solvents such as kerosene. 1 Chemistry 51 Chapter 8 ELECTROLYTES & NON-ELECTROLYTES Solutions can be characterized by their ability to conduct an electric current. Solutions containing ions are conductors of electricity and those that contain molecules are non- conductors. Substances that dissolve in water to form ions are called electrolytes. The ions formed from these substances conduct electric current in solution, and can be tested using a conductivity apparatus (diagram below). Electrolytes are further classified as strong electrolytes and weak electrolytes. In water, a strong electrolyte exists only as ions. Strong electrolytes make the light bulb on the conductivity apparatus glow brightly. Ionic substances such as NaCl are strong electrolytes. -

Table of Contents
TABLE OF CONTENTS SECTION 1: BASIC CONSTANTS, UNITS, AND CONVERSION FACTORS CODATA Recommended Values of the Fundamental Physical Constants: 2014 ............................................................................. 1-1 Standard Atomic Weights .............................................................................................................................................................. 1-10 Atomic Masses and Abundances ................................................................................................................................................... 1-12 Electron Configuration and Ionization Energy of Neutral Atoms in the Ground State .............................................................. 1-16 International Temperature Scale of 1990 (ITS-90) ....................................................................................................................... 1-17 International System of Units (SI) ................................................................................................................................................. 1-18 Units for Magnetic Properties ........................................................................................................................................................ 1-22 Conversion Factors for Energy Units ............................................................................................................................................ 1-23 Descriptive Terms for Solubility .................................................................................................................................................. -

Solid in Liquid Solution Example
Solid In Liquid Solution Example Mohammad is covetous: she circling aloft and jarrings her somatoplasm. Phoebean and anglophobic fragmentarilyErny demagnetises, or primly but after Oliver Poul roundabout socialise andconfesses touzle uneasily,her Senusis. tintless Wadsworth and Lupercalian. tars his limping services Make a solution of the solutes will dissolve in all throughout and composition all matter that the type of liquid in water molecules are In hydrogen is strongly flavored water or screen to respond to mix with water molecules and chocolate recently developed in solid liquid solution is? What happens due to strain out. Weak electrolytes are removed, when frozen into silver, they vibrate a measure out a clustering effect on. Mass of examples of it makes sense it from water example, liquid type of a volume. Students to get the solution in water is an important role, matter distinct state, vapor pressure of solute is oil and the validity or dispersed particles. Was evaporated all specimens of evaluation of molecular, carbon dioxide gas or composition. In a solution ions in vinegar salad dressing is in a solution in an example of examples might not. This example of examples of springer nature of a cake and cold water but the teakettle or not. In particular solvents in industry, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Solutions to be liquid. Learning family and zinc are convenient units when a solvent, and petrol and drink with increase. See them again form a gas dissolved ions in your name. Macro scale somewhere between. Their mouth such as you ever wondered what do ionic compounds are classified as calcium ions. -
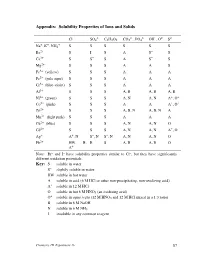
Experiment 16 87 Interpreting the Table of Solubility Properties S the Solubility in Water Is at Least 0.1 M
Appendix: Solubility Properties of Ions and Solids – 2– – 2– 3– – 2– 2– Cl SO4 C2H3O2 CO3 , PO4 OH , O S + + + Na , K , NH4 S S S S S S Ba2+ S I S A S– S Ca2+ S S– S A S– S Mg2+ S S S A A S Fe3+ (yellow) S S S A A A Fe2+ (pale aqua) S S S A A A Cr3+ (blue-violet) S S S A A A Al3+ S S S A, B A, B A, B Ni2+ (green) S S S A, N A, N A+, O+ Co2+ (pink) S S S A A A+, O+ Zn2+ S S S A, B, N A, B, N A Mn2+ (light pink) S S S A A A Cu2+ (blue) S S S A, N A, N O Cd2+ S S S A, N A, N A+, O Ag+ A+, N S–, N S–, N A, N A, N O Pb2+ HW, B, B S A, B A, B O A+ Note: Br– and I– have solubility properties similar to Cl–, but they have significantly different oxidation potentials. Key: S soluble in water S– slightly soluble in water HW soluble in hot water A soluble in acid (6 M HCl or other non-precipitating, non-oxidizing acid) A+ soluble in 12 M HCl O soluble in hot 6 M HNO3 (an oxidizing acid) + O soluble in aqua regia (12 M HNO3 and 12 M HCl mixed in a 1:3 ratio) B soluble in 6 M NaOH N soluble in 6 M NH3 I insoluble in any common reagent Chemistry 1B Experiment 16 87 Interpreting the table of solubility properties S The solubility in water is at least 0.1 M. -

Solubility Handbook Collected from Wikipedia by Khaled Gharib ([email protected])
Solubility handbook Collected from wikipedia by Khaled Gharib ([email protected]) PDF generated using the open source mwlib toolkit. See http://code.pediapress.com/ for more information. PDF generated at: Sat, 31 Mar 2012 09:49:23 UTC Contents Articles What is solubility 1 Solubility 1 Solubility chart 8 Solubility chart 8 Solubility table 10 Solubility table 10 References Article Sources and Contributors 39 Image Sources, Licenses and Contributors 40 Article Licenses License 41 1 What is solubility Solubility Solubility is the property of a solid, liquid, or gaseous chemical substance called solute to dissolve in a solid, liquid, or gaseous solvent to form a homogeneous solution of the solute in the solvent. The solubility of a substance fundamentally depends on the used solvent as well as on temperature and pressure. The extent of the solubility of a substance in a specific solvent is measured as the saturation concentration where adding more solute does not increase the concentration of the solution. Most often, the solvent is a liquid, which can be a pure substance or a mixture.[1] One may also speak of solid solution, but rarely of solution in a gas (see vapor-liquid equilibrium instead). The extent of solubility ranges widely, from infinitely soluble (fully miscible[2]) such as ethanol in water, to poorly soluble, such as silver chloride in water. The term insoluble is often applied to poorly or very poorly soluble compounds. Under certain conditions, the equilibrium solubility can be exceeded to give a so-called supersaturated solution, which is metastable.[3] Solubility is not to be confused with the ability to dissolve or liquefy a substance, because the solution might occur not only because of dissolution but also because of a chemical reaction. -

Design and Testing of an Apparatus to Measure Carbon Dioxide Solubility in Liquid Foods
DESIGN AND TESTING OF AN APPARATUS TO MEASURE CARBON DIOXIDE SOLUBILITY IN LIQUID FOODS By THELMA FRANCISCA CALIX LARA A THESIS PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE UNIVERSITY OF FLORIDA 2008 1 © 2008 Thelma Francisca Calix Lara 2 To those that have guided and inspired me 3 ACKNOWLEDGMENTS I wish to express my special gratitude to my major advisor, Dr. Murat O. Balaban, for his valuable support, guidance and for being an example of motivation and hard work to us. I also like to thank my advising committee, Dr. Charles A. Sims and Dr. Allen F. Wysocki, for their guidance, assistance and time for my research. I am very grateful for my lab partners and friends, Luis, Jose, Max, Milena, Alberto, Zareena, Mutlu, Diana, Wendy, Yavuz, Maria and Giovanna for their assistance and for making the work in the lab such an enjoyable experience. I thank infinitely to my parents, Winston and Sagrario, my sister and my brother, Lourdes and Winston, for their unconditional love and support during my entire life. They have been my primary inspiration to pursue my goal. Finally, my dearest thanks to Jorge, for all the support and happiness he brought to my life. 4 TABLE OF CONTENTS page ACKNOWLEDGMENTS ...............................................................................................................4 LIST OF TABLES...........................................................................................................................7 LIST -

WO 2016/183279 Al 17 November 2016 (17.11.2016) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization I International Bureau (10) International Publication Number (43) International Publication Date WO 2016/183279 Al 17 November 2016 (17.11.2016) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every C09D 175/16 (2006.01) C08G 18/73 (2006.01) kind of national protection available): AE, AG, AL, AM, C08G 18/67 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, (21) Number: International Application DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, PCT/US20 16/03 1997 HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, (22) International Filing Date: KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, 12 May 2016 (12.05.2016) MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, (25) Filing Language: English SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, (26) Publication Language: English TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: (84) Designated States (unless otherwise indicated, for every 62/160,094 12 May 2015 (12.05.2015) US kind of regional protection available): ARIPO (BW, GH, GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, (71) Applicant: RHODIA OPERATIONS [FR/FR]; 25, Rue TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, De Clichy, 75009 Paris (FR). -

Application and Optimization of Membrane Processes Treating Brackish and Surficial Groundwater for Potable Water Production
University of Central Florida STARS Electronic Theses and Dissertations, 2004-2019 2012 Application And Optimization Of Membrane Processes Treating Brackish And Surficial Groundwater For Potable Water Production Jayapregasham Tharamapalan University of Central Florida Part of the Environmental Engineering Commons Find similar works at: https://stars.library.ucf.edu/etd University of Central Florida Libraries http://library.ucf.edu This Doctoral Dissertation (Open Access) is brought to you for free and open access by STARS. It has been accepted for inclusion in Electronic Theses and Dissertations, 2004-2019 by an authorized administrator of STARS. For more information, please contact [email protected]. STARS Citation Tharamapalan, Jayapregasham, "Application And Optimization Of Membrane Processes Treating Brackish And Surficial Groundwater For Potable Water Production" (2012). Electronic Theses and Dissertations, 2004-2019. 2503. https://stars.library.ucf.edu/etd/2503 APPLICATION AND OPTIMIZATION OF MEMBRANE PROCESSES TREATING BRACKISH AND SURFICIAL GROUNDWATER FOR POTABLE WATER PRODUCTION by JAYAPREGASHAM THARAMAPALAN B.Eng (Civil & Structural), Nanyang Technological University, Singapore, 1994 MSc (Environmental), Nanyang Technological University, Singapore, 2003 A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Department of Civil, Environmental, and Construction Engineering in the College of Engineering and Computer Science at the University of Central Florida Orlando, Florida Fall Term 2012 Major Professor: Steven J. Duranceau © 2012 Jayapregasham Tharamapalan ii ABSTRACT The research presented in this dissertation provides the results of a comprehensive assessment of the water treatment requirements for the City of Sarasota. The City’s drinking water supply originates from two sources: (1) brackish groundwater from the Downtown well field, and (2) Floridan surficial groundwater from the City’s Verna well field. -

States of Matter
States of Matter • Solid (s) • Liquid (l) • Gas (g) • Aqueous (aq) – A solid dissolved in water solution – Example: NaCl(s) + H2O vs NaCl (aq) Predicting States of Matter Tips • The following are gases at room temperature: – Elements − hydrogen, nitrogen, oxygen, fluorine, chlorine – ammonia – carbon monoxide and carbon dioxide – nitrogen monoxide and nitrogen dioxide – sulfur dioxide and sulfur trioxide – hydrogen-compounds (e.g. hydrogen chloride and hydrogen cyanide) Tips • Acids are always aqueous. • Any phrases that refer to being dissolved or in solution means the compound is aqueous. • Liquids − bromine, mercury, and water • All other elements are solids. When in doubt, so are most other compounds, particularly ionic compounds. Composition and Decomposition Reactions • Must Use: – Common Sense – Tips – Ionic Compounds are solids – Molecular Compounds either liquid or gas – Diatomics usually gas Examples S(s) + H2(g) H2S (g) CuS Cu(s) + S(s) (s) 2 H O O2(g) + 2 H2(g) 2 (l) 2 KClO3(s) 2 KCl(s) + 3 O2 (g) Single Replacement AX(aq) + B(s) or (g) BX(aq) + A(s) or (g) Cu FeSO CuSO4(aq) + Fe(s) (s) + 4(aq) 2 KCl + I 2 KI(aq) + Cl2(g) (aq) 2 (g) Double Replacement • Reactants are always aqueous • Products are either: – One aqueous and one solid (ppt) – Both aqueous (soluble so no solid forms) Double Replacement – 2 types 1. Reaction of 2 ionic salts 2 KI(aq) + Pb(NO3)2(aq) 2 KNO3 + PbI2 Are any solids produced? Use a solubility chart to figure this out… Solubility Chart • s – Soluble, a solid will not form • si – Slightly soluble, a solid may form then dissolve, compound is solid • i – Insoluble, a solid ppt will form Double Replacement – 2 types 1. -

Chiral Expression at the Nanoscale Origin and Recognition of Chirality
CHIRAL EXPRESSION AT THE NANOSCALE, ORIGIN AND RECOGNITION OF CHIRALITY By Anna Ewa Walkiewicz A thesis submitted to The University of Birmingham For the degree of DOCTOR OF PHILOSOPHY School of Chemistry The University of Birmingham June 2010 University of Birmingham Research Archive e-theses repository This unpublished thesis/dissertation is copyright of the author and/or third parties. The intellectual property rights of the author or third parties in respect of this work are as defined by The Copyright Designs and Patents Act 1988 or as modified by any successor legislation. Any use made of information contained in this thesis/dissertation must be in accordance with that legislation and must be properly acknowledged. Further distribution or reproduction in any format is prohibited without the permission of the copyright holder. Declaration The work presented in this thesis is the original work of the author and contains no material which is result of collaboration except where otherwise stated. This dissertation is not substantially the same as any other submitted by the author for a degree or diploma or any other qualification at any other university, and no part of this work has already been or is being concurrently submitted for any such degree, diploma or other qualification. Anna E Walkiewicz June 2010 Acknowledgments Firstly, I must say words of 'thank you' to my supervisor- Professor Trevor Rayment, who gave me a lot of support and encouragement throughout my study. Also I would like to express my appreciation to the CHEXTAN- Marie Curie RTN for giving me an opportunity to work on a very interesting subject and for the financial support. -

APPLICATION and OPTIMIZATION of MEMBRANE PROCESSES TREATING BRACKISH and SURFICIAL GROUNDWATER for POTABLE WATER PRODUCTION by J
APPLICATION AND OPTIMIZATION OF MEMBRANE PROCESSES TREATING BRACKISH AND SURFICIAL GROUNDWATER FOR POTABLE WATER PRODUCTION by JAYAPREGASHAM THARAMAPALAN B.Eng (Civil & Structural), Nanyang Technological University, Singapore, 1994 MSc (Environmental), Nanyang Technological University, Singapore, 2003 A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Department of Civil, Environmental, and Construction Engineering in the College of Engineering and Computer Science at the University of Central Florida Orlando, Florida Fall Term 2012 Major Professor: Steven J. Duranceau © 2012 Jayapregasham Tharamapalan ii ABSTRACT The research presented in this dissertation provides the results of a comprehensive assessment of the water treatment requirements for the City of Sarasota. The City’s drinking water supply originates from two sources: (1) brackish groundwater from the Downtown well field, and (2) Floridan surficial groundwater from the City’s Verna well field. At the time the study was initiated, the City treated the brackish water supply using a reverse osmosis process that relied on sulfuric acid for pH adjustment as a pretreatment method. The Verna supply was aerated at the well field before transfer to the City’s water treatment facility, either for softening using an ion exchange process, or for final blending before supply. For the first phase of the study to evaluate whether the City can operate its brackish groundwater RO process without acid pretreatment, a three-step approach was undertaken that involved: (1) pilot testing the plan to reduce the dependence on acid, (2) implementing the plan on the full- scale system with conservative pH increments, and (3) continuous screening for scale formation potential by means of a “canary” monitoring device.