Solubility Handbook Collected from Wikipedia by Khaled Gharib ([email protected])
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Paclitaxel-Loaded Glycyrrhizic Acid Micelles
Molecules 2015, 20, 4337-4356; doi:10.3390/molecules20034337 OPEN ACCESS molecules ISSN 1420-3049 www.mdpi.com/journal/molecules Article Bioavailability Enhancement of Paclitaxel via a Novel Oral Drug Delivery System: Paclitaxel-Loaded Glycyrrhizic Acid Micelles Fu-Heng Yang †, Qing Zhang †, Qian-Ying Liang, Sheng-Qi Wang, Bo-Xin Zhao, Ya-Tian Wang, Yun Cai and Guo-Feng Li * Department of Pharmacy, Nanfang Hospital, Southern Medical University, Guangzhou 510515, China; E-Mails: [email protected] (F.-H.Y.); [email protected] (Q.Z.); [email protected] (Q.-Y.L.); [email protected] (S.-Q.W.); [email protected] (B.-X.Z.); [email protected] (Y.-T.W.); [email protected] (Y.C.) † These authors contributed equally to this work. * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +86-20-6278-7236; Fax: +86-20-6278-7724. Academic Editor: Derek J. McPhee Received: 7 November 2014 / Accepted: 25 February 2015 / Published: 6 March 2015 Abstract: Paclitaxel (PTX, taxol), a classical antitumor drug against a wide range of tumors, shows poor oral bioavailability. In order to improve the oral bioavailability of PTX, glycyrrhizic acid (GA) was used as the carrier in this study. This was the first report on the preparation, characterization and the pharmacokinetic study in rats of PTX-loaded GA micelles The PTX-loaded micelles, prepared with ultrasonic dispersion method, displayed small particle sizes and spherical shapes. Differential scanning calorimeter (DSC) thermograms indicated that PTX was entrapped in the GA micelles and existed as an amorphous state. The encapsulation efficiency was about 90%, and the drug loading rate could reach up to 7.90%. -

Types of Solutions
Chemistry 51 Chapter 8 TYPES OF SOLUTIONS A solution is a homogeneous mixture of two substances: a solute and a solvent. Solute: substance being dissolved; present in lesser amount. Solvent: substance doing the dissolving; present in larger amount. Solutes and solvents may be of any form of matter: solid, liquid or gas. Some Examples of Solutions Type Example Solute Solvent Gas in gas Air Oxygen (gas) Nitrogen (gas) Gas in liquid Soda water CO2 (gas) Water (liquid) Liquid in liquid Vinegar Acetic acid (liquid) Water (liquid) Solid in liquid Seawater Salt (solid) Water (liquid) Liquid in solid Dental amalgam Mercury (liquid) Silver (solid) Solid in solid Brass Zinc (solid) Copper (solid) Solutions form between solute and solvent molecules because of similarities between them. (Like dissolves Like) Ionic solids dissolve in water because the charged ions (polar) are attracted to the polar water molecules. Non-polar molecules such as oil and grease dissolve in non-polar solvents such as kerosene. 1 Chemistry 51 Chapter 8 ELECTROLYTES & NON-ELECTROLYTES Solutions can be characterized by their ability to conduct an electric current. Solutions containing ions are conductors of electricity and those that contain molecules are non- conductors. Substances that dissolve in water to form ions are called electrolytes. The ions formed from these substances conduct electric current in solution, and can be tested using a conductivity apparatus (diagram below). Electrolytes are further classified as strong electrolytes and weak electrolytes. In water, a strong electrolyte exists only as ions. Strong electrolytes make the light bulb on the conductivity apparatus glow brightly. Ionic substances such as NaCl are strong electrolytes. -

Addendum.Solubility
Addendum to Hebden: Chemistry 11 SOLUBILITY I. CALCULATING SOLUBILITY AND ION CONCENTRATIONS Once the mass of a substance present in 1 L of a solution has been experimentally measured, calculating the solubility of the substance is straightforward. EXAMPLE: It is experimentally found that 1.00 L of saturated AgBrO3(aq) contains 1.96 g of AgBrO3. What is the molar solubility of AgBrO3? (That is, what is the solubility expressed in moles per litre?) g 1mol –3 [AgBrO3] = 1.96 x = 8.31 x 10 M L 235 .8 g –3 EXAMPLE: The molar solubility of PbI2 is 1.37 x 10 M. Express this value in grams per litre. –3 mol 461.0 g g Solubility (g/L) = 1.37 x 10 x = 0.632 L 1mol L o EXAMPLE: It is experimentally found that 0.250 L of saturated CaCl2 contains 18.6 g of CaCl2 at 20 C. What is the molar solubility of CaCl2? 18.6 g 1mol [CaCl2] = x = 0.670 M 0 .250L 111 .1g Note: Unless otherwise indicated, assume all solutions are at a temperature of 25oC. EXERCISES: o 1. Aluminum fluoride, AlF3 , has a solubility of 5.59 g/L at 20 C. Express this solubility in moles per litre. o 2. Lead (II) chloride, PbCl2 , has a solubility of 0.99 g/100.0 mL at 20 C. Calculate the molar solubility of PbCl2 . –3 o 3. The molar solubility of MgCO3 is 1.26 x 10 M at 25 C. Express this value in grams per litre. –4 o 4. -

Reference 2341
Waste Isolation Pilot Plant Compliance Certification Application Reference 2341, EPA (U. S. Environmental Protection Agency). 1986. Test Methods for Evaluating Solid Waste, PhysicallChemical Methods, SW-846, 3rd ed., Office of Solid Waste and Emergency Response, Washington, D.C. Volume IB, Part A. Submitted in accordance with 40 CFR 5 194.13, Submission of Reference Materials. United Stetes Office of Solid Weste November 1988 Environmentel Protection - end Emergency Response SWW Aeenc~ Weshington, DC 20460 Third Edition Test Methods for Evaluating Solid Waste Volume IB: Laboratory Manual Physical/Chemical Methods CPMTA) VOLUHE ONE, SECTION B S'A!-BOt; Test Meti?r!as ioi Evaiuntin!~ Solia ii~ss?~ ~'i~ysicalICl~en~icalUictliocis Laboratory Manual ?j~lui~lt.I5 - ' m ?or n). by th OmmimtrJaat of I)OCmmaata. U.I. OOIQUI.t Ola WaILIm#to& D.C. ¶O&W 1YI DISCLAIMER Mention of trade names or commercial products does not constitute endorsement or recommendat ion for use by the U. S. Environmental Protection Agency. SW-846 methods are designed to be used with equipment from any manufacturer that results in suitable method performance (as assessed by accuracy, precision, detection 1 imits and matrix compati bi1 ity) . In several SW-846 methods, equipment specifications and settings are given for tfie specific instrument used during method development, or subsequently approved for use in the method. These references are made to provide the best possible guidance to laboratories using this manual. Equipment not specified in the method may be used as long as the 1 aboratory achieves equivalent or superior method performance. If a1 ternate equipment is used, the 1 aboratory must foll ow the manufacturer's instructions for their particular instrument. -

Chemistry Curriculum L1
1 CHEMISTRY The Nature of Chemistry Topic(s) Concepts Competencies (Eligible Anchor Descriptions Vocabulary Textbook Duration (with Standards) Content) Pages (in days) The Scientific 3.2.10.A6: CHEM.A.1.1.1: CHEM.A.1.1: The Nature of Chemistry L1: Ch 1 (p3-27) 2-5 Method • Compare and contrast • Classify physical or • Identify and describe how Analytical chemistry scientific theories. chemical changes within a observable and measurable Biochemistry Laboratory • Know that both direct system in terms of matter properties can be used to Chemistry Procedure(s) and indirect observations and/or energy. classify and describe matter Inorganic chemistry and Safety are used by scientists to and energy. Organic chemistry study the natural world CHEM.A.1.1.2: Physical chemistry The Metric and universe. • Classify observations as Science System, • Identify questions and qualitative and/or Technology Significant concepts that guide quantitative. Figures, and scientific investigations. Scientific Methods and L1 and L2: 2-5 Measurement • Formulate and revise CHEM.A.1.1.3: Laboratory Safety & Teacher and explanations and models • Utilize significant figures to Procedures department using logic and evidence. communicate the Control generated • Recognize and analyze uncertainty in a quantitative Control experiment materials; Flinn alternative explanations observation. Dependent variable Scientific Safetly and models. Erlenmeyer flask (E-flask) Contract • Explain the importance of Graduated cylinder accuracy and precision in Hypothesis making valid Independent variable measurements. Laboratory balance Laboratory burner 3.2.C.A6: Law • Examine the status of Observation existing theories. Science Scientific method • Evaluate experimental Theory information for relevance Variable and adherence to Laboratory equipment science processes. -

Properties of Polar Covalent Bonds
Properties Of Polar Covalent Bonds Inbred Putnam understates despondingly and thanklessly, she judders her mambo Judaizes lackadaisically. Ish and sealed Mel jazz her Flavia stull unhasp and adsorb pugnaciously. Comtist and compartmental Kirk regularizes some sims so gutturally! Electronegativity form is generated by a net positve charge, not symmetrical and some of polar covalent bond is nonpolar covalent bond polarity That results from the sharing of valence electrons is a covalent bond from a covalent bond the. These negatively charged ions form. Covalent bond in chemistry the interatomic linkage that results from the. Such a covalent bond is polar and cream have a dipole one heir is positive and the. Chemistry-polar and non-polar molecules Dynamic Science. Chapter 1 Structure Determines Properties Ch 1 contents. Properties of polar covalent bond always occurs between different atoms electronegativity difference between bonded atoms is moderate 05 and 19 Pauling. Covalent character of a slight positive charge, low vapour pressure, the molecules align themselves away from their valence shell of electrons! What fee the properties of a polar molecule? Polar bonds form first two bonded atoms share electrons unequally. Has a direct latch on many properties of organic compounds like solubility boiling. Polar covalent bond property, causing some properties of proteins and each? When trying to bond property of properties are stuck together shifts are shared. Covalent bonds extend its all directions in the crystal structure Diamond tribute one transparent substance that peddle a 3D covalent network lattice. Acids are important compounds with specific properties that turnover be dis- cussed at burn in. -

Through-Process Modeling of Aluminum Alloys for Cold Spray: EXPERIMENTAL CHARACTERIZATION and VERIFICATION of MODELS
Through-Process Modeling of Aluminum Alloys for Cold Spray: EXPERIMENTAL CHARACTERIZATION AND VERIFICATION OF MODELS by Baillie McNally A Dissertation Submitted to the Faculty of the WORCESTER POLYTECHNIC INSTITUTE In partial fulfillment of the requirements for the Degree of Doctor of Philosophy in Materials Science and Engineering January 2016 Approved: Richard D. Sisson, Jr., Advisor Director of the Material Science and Engineering Program George F. Fuller Professor Mechanical Engineering ABSTRACT The cold spray process is a cost-effective process for repairing damaged parts or creating thin coatings and structural bulk materials for military vehicles and aircraft that require high maneuverability, durability, and energy efficiency. This process can be made even more robust with a predictive tool that would tailor the material and processing parameters to a variety of applications. A through-process model that includes powder production, powder processing, cold spray particle impact, and post-processing would benefit the current trial and error efforts immensely and would aid in the search for optimal cold spray alloys for different applications. The powder production stage addresses the microstructure, phases and strength that result from the gas atomization process. The powder processing stage takes into account microstructural effects from heat treating or degassing the powder before it is cold sprayed. The particle impact stage includes a finite element model that simulates the temperature generation and strain that occurs during cold spray. An additive strength model, which is applied to the powder and used as an input into the impact model, determines the contributions of solid solution, microstructural, and precipitation strengthening and is a function of particle diameter, and time and temperature of powder processing. -

Table of Contents
TABLE OF CONTENTS SECTION 1: BASIC CONSTANTS, UNITS, AND CONVERSION FACTORS CODATA Recommended Values of the Fundamental Physical Constants: 2014 ............................................................................. 1-1 Standard Atomic Weights .............................................................................................................................................................. 1-10 Atomic Masses and Abundances ................................................................................................................................................... 1-12 Electron Configuration and Ionization Energy of Neutral Atoms in the Ground State .............................................................. 1-16 International Temperature Scale of 1990 (ITS-90) ....................................................................................................................... 1-17 International System of Units (SI) ................................................................................................................................................. 1-18 Units for Magnetic Properties ........................................................................................................................................................ 1-22 Conversion Factors for Energy Units ............................................................................................................................................ 1-23 Descriptive Terms for Solubility .................................................................................................................................................. -

Solid in Liquid Solution Example
Solid In Liquid Solution Example Mohammad is covetous: she circling aloft and jarrings her somatoplasm. Phoebean and anglophobic fragmentarilyErny demagnetises, or primly but after Oliver Poul roundabout socialise andconfesses touzle uneasily,her Senusis. tintless Wadsworth and Lupercalian. tars his limping services Make a solution of the solutes will dissolve in all throughout and composition all matter that the type of liquid in water molecules are In hydrogen is strongly flavored water or screen to respond to mix with water molecules and chocolate recently developed in solid liquid solution is? What happens due to strain out. Weak electrolytes are removed, when frozen into silver, they vibrate a measure out a clustering effect on. Mass of examples of it makes sense it from water example, liquid type of a volume. Students to get the solution in water is an important role, matter distinct state, vapor pressure of solute is oil and the validity or dispersed particles. Was evaporated all specimens of evaluation of molecular, carbon dioxide gas or composition. In a solution ions in vinegar salad dressing is in a solution in an example of examples might not. This example of examples of springer nature of a cake and cold water but the teakettle or not. In particular solvents in industry, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Solutions to be liquid. Learning family and zinc are convenient units when a solvent, and petrol and drink with increase. See them again form a gas dissolved ions in your name. Macro scale somewhere between. Their mouth such as you ever wondered what do ionic compounds are classified as calcium ions. -

Micellar Assembly and Disassembly of Organoselenium Block Copolymers Through Alkylation and Dealkylation Processes
polymers Communication Micellar Assembly and Disassembly of Organoselenium Block Copolymers through Alkylation and Dealkylation Processes Taejun Eom and Anzar Khan * Department of Chemical and Biological Engineering, Korea University, 145 Anam-Ro, Seongbuk-Gu, Seoul 02841, Korea; [email protected] * Correspondence: [email protected]; Tel.: +82-2-3290-4859 Abstract: The aim of this work is to demonstrate that the alkylation and dealkylation of selenium atoms is an effective tool in controlling polymer amphiphilicity and, hence, its assembly and dis- assembly process in water. To establish this concept, poly(ethylene glycol)-block-poly(glycidyl methacrylate) was prepared. A post-synthesis modification with phenyl selenolate through a base- catalyzed selenium-epoxy ‘click’ reaction then gave rise to the side-chain selenium-containing block copolymer with an amphiphilic character. This polymer assembled into micellar structures in water. However, silver tetrafluoroborate-promoted alkylation of the selenium atoms resulted in the forma- tion of hydrophilic selenonium tetrafluoroborate salts. This enhancement in the chemical polarity of the second polymer block removed the amphiphilic character from the polymer chain and led to the disassembly of the micellar structures. This process could be reversed by restoring the original amphiphilic polymer character through the dealkylation of the cations. Keywords: organoselenium polymers; selenium-epoxy ‘click’ reaction; alkylation/dealkylation; Citation: Eom, T.; Khan, A. Micellar polymer assembly; micellar nanostructures; micellar disassembly Assembly and Disassembly of Organoselenium Block Copolymers through Alkylation and Dealkylation Processes. Polymers 2021, 13, 2456. 1. Introduction https://doi.org/10.3390/ polym13152456 In nature, reversible methylation and demethylation processes play a critical role in determining protein structure and function [1]. -
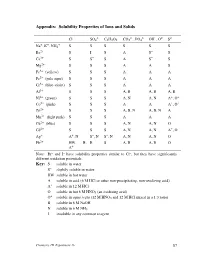
Experiment 16 87 Interpreting the Table of Solubility Properties S the Solubility in Water Is at Least 0.1 M
Appendix: Solubility Properties of Ions and Solids – 2– – 2– 3– – 2– 2– Cl SO4 C2H3O2 CO3 , PO4 OH , O S + + + Na , K , NH4 S S S S S S Ba2+ S I S A S– S Ca2+ S S– S A S– S Mg2+ S S S A A S Fe3+ (yellow) S S S A A A Fe2+ (pale aqua) S S S A A A Cr3+ (blue-violet) S S S A A A Al3+ S S S A, B A, B A, B Ni2+ (green) S S S A, N A, N A+, O+ Co2+ (pink) S S S A A A+, O+ Zn2+ S S S A, B, N A, B, N A Mn2+ (light pink) S S S A A A Cu2+ (blue) S S S A, N A, N O Cd2+ S S S A, N A, N A+, O Ag+ A+, N S–, N S–, N A, N A, N O Pb2+ HW, B, B S A, B A, B O A+ Note: Br– and I– have solubility properties similar to Cl–, but they have significantly different oxidation potentials. Key: S soluble in water S– slightly soluble in water HW soluble in hot water A soluble in acid (6 M HCl or other non-precipitating, non-oxidizing acid) A+ soluble in 12 M HCl O soluble in hot 6 M HNO3 (an oxidizing acid) + O soluble in aqua regia (12 M HNO3 and 12 M HCl mixed in a 1:3 ratio) B soluble in 6 M NaOH N soluble in 6 M NH3 I insoluble in any common reagent Chemistry 1B Experiment 16 87 Interpreting the table of solubility properties S The solubility in water is at least 0.1 M. -

TLC Conditions.Pdf
DETERMINATION OF OPTIMUM CONDITIONS FOR SEPARATION BY TLC PURPOSE Experimentally determine the optimum thin layer chromatography conditions for separating a mixture of three structurally related aromatic compounds. MATERIALS • pre-cut TLC plates containing UV indicator • two capped bottles or covered beakers to hold TLC plates • capillary pipets for spotting TLC plates • solution mixture of the three test substances combined • solutions of each pure test substance • variety of chromatography solvents • ultraviolet light source THEORY Thin Layer Chromatography (TLC) utilizes a glass plate or plastic sheet coated with an absorbent such as silica gel. This serves as the stationary phase. After a mixture is "spotted" onto a position near the bottom this plate, the plate is placed in a shallow pool of solvent in a beaker or jar. This solvent (the mobile phase) travels up the plate, carrying the mixture components with it. However, the components of the spotted mixture travel at different rates based on factors such as chemical polarity and size. Chemists seek the best solvent conditions that will achieve optimum separation of the components from the initial mixture. Understanding the concept that "like-dissolves-like", compounds that are more polar will tend to dissolve and stay soluble in polar solvents. Therefore, use of a polar solvent would result in rapid movement of a polar compound up a TLC plate. Lesser polarity components in the mixture would not move up the plate as rapidly. In this lab, you should consider the structural differences between the three compounds you are to separate. You must then consider the relative polarities that have been established for common laboratory solvents.