States of Matter
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Paclitaxel-Loaded Glycyrrhizic Acid Micelles
Molecules 2015, 20, 4337-4356; doi:10.3390/molecules20034337 OPEN ACCESS molecules ISSN 1420-3049 www.mdpi.com/journal/molecules Article Bioavailability Enhancement of Paclitaxel via a Novel Oral Drug Delivery System: Paclitaxel-Loaded Glycyrrhizic Acid Micelles Fu-Heng Yang †, Qing Zhang †, Qian-Ying Liang, Sheng-Qi Wang, Bo-Xin Zhao, Ya-Tian Wang, Yun Cai and Guo-Feng Li * Department of Pharmacy, Nanfang Hospital, Southern Medical University, Guangzhou 510515, China; E-Mails: [email protected] (F.-H.Y.); [email protected] (Q.Z.); [email protected] (Q.-Y.L.); [email protected] (S.-Q.W.); [email protected] (B.-X.Z.); [email protected] (Y.-T.W.); [email protected] (Y.C.) † These authors contributed equally to this work. * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +86-20-6278-7236; Fax: +86-20-6278-7724. Academic Editor: Derek J. McPhee Received: 7 November 2014 / Accepted: 25 February 2015 / Published: 6 March 2015 Abstract: Paclitaxel (PTX, taxol), a classical antitumor drug against a wide range of tumors, shows poor oral bioavailability. In order to improve the oral bioavailability of PTX, glycyrrhizic acid (GA) was used as the carrier in this study. This was the first report on the preparation, characterization and the pharmacokinetic study in rats of PTX-loaded GA micelles The PTX-loaded micelles, prepared with ultrasonic dispersion method, displayed small particle sizes and spherical shapes. Differential scanning calorimeter (DSC) thermograms indicated that PTX was entrapped in the GA micelles and existed as an amorphous state. The encapsulation efficiency was about 90%, and the drug loading rate could reach up to 7.90%. -

Types of Solutions
Chemistry 51 Chapter 8 TYPES OF SOLUTIONS A solution is a homogeneous mixture of two substances: a solute and a solvent. Solute: substance being dissolved; present in lesser amount. Solvent: substance doing the dissolving; present in larger amount. Solutes and solvents may be of any form of matter: solid, liquid or gas. Some Examples of Solutions Type Example Solute Solvent Gas in gas Air Oxygen (gas) Nitrogen (gas) Gas in liquid Soda water CO2 (gas) Water (liquid) Liquid in liquid Vinegar Acetic acid (liquid) Water (liquid) Solid in liquid Seawater Salt (solid) Water (liquid) Liquid in solid Dental amalgam Mercury (liquid) Silver (solid) Solid in solid Brass Zinc (solid) Copper (solid) Solutions form between solute and solvent molecules because of similarities between them. (Like dissolves Like) Ionic solids dissolve in water because the charged ions (polar) are attracted to the polar water molecules. Non-polar molecules such as oil and grease dissolve in non-polar solvents such as kerosene. 1 Chemistry 51 Chapter 8 ELECTROLYTES & NON-ELECTROLYTES Solutions can be characterized by their ability to conduct an electric current. Solutions containing ions are conductors of electricity and those that contain molecules are non- conductors. Substances that dissolve in water to form ions are called electrolytes. The ions formed from these substances conduct electric current in solution, and can be tested using a conductivity apparatus (diagram below). Electrolytes are further classified as strong electrolytes and weak electrolytes. In water, a strong electrolyte exists only as ions. Strong electrolytes make the light bulb on the conductivity apparatus glow brightly. Ionic substances such as NaCl are strong electrolytes. -

Ionic Liquid and Supercritical Fluid Hyphenated Techniques for Dissolution and Separation of Lanthanides, Actinides, and Fission Products
Project No. 09-805 Ionic Liquid and Supercritical Fluid Hyphenated Techniques for Dissolution and Separation of Lanthanides, Actinides, and Fission Products ItIntegrat tdUied Universit itPy Programs Dr. Chien Wai University of Idaho In collaboration with: Idaho National Laboratory Jack Law, Technical POC James Bresee, Federal POC 1 Ionic Liquid and Supercritical Fluid Hyphenated Techniques For Dissolution and Separation of Lanthanides and Actinides DOE-NEUP Project (TO 00058) Final Technical Report Principal Investigator: Chien M. Wai Department of Chemistry, University of Idaho, Moscow, Idaho 83844 Date: December 1, 2012 2 Table of Contents Project Summary 3 Publications Derived from the Project 5 Chapter I. Introduction 6 Chapter II. Uranium Dioxide in Ionic Liquid with a TP-HNO3 Complex – Dissolution and Coordination Environment 9 1. Dissolution of UO2 in Ionic Liquid with TBP(HNO3)1.8(H2O)0.6 2. Raman Spectra of Dissolved Uranyl Species in IL 13 3. Transferring Uranium from IL Phase to sc-CO2 15 Chapter III. Kinetic Study on Dissolution of Uranium Dioxide and Neodymium Sesquioxide in Ionic Liquid 19 1. Rate of Dissolution of UO2 and Nd2O3 in RTIL 19 2. Temperature Effect on Dissolution of UO2 and Nd2O3 24 3. Viscosity Effect on Dissolution of UO2 in IL with TBP(HNO3)1.8(H2O)0.6 Chapter IV. Separation of UO2(NO3)2(TBP)2 and Nd(NO3)3(TBP)3 in Ionic Liquid Using Diglycolamide and Supercritical CO2 Extraction 30 1. Complexation of Uranyl with Diglycolamide TBDGA in Ionic Liquid 31 2. Complexation of Neodymium(III) with TBDGA in Ionic Liquid 35 3. Solubility and Distribution Ratio of UO2(NO3)2(TBP)2 and Nd(NO3)3(TBP)3 in Supercritical CO2 Phase 38 4. -

Application of Supercritical Fluids Review Yoshiaki Fukushima
1 Application of Supercritical Fluids Review Yoshiaki Fukushima Abstract Many advantages of supercritical fluids come Supercritical water is expected to be useful in from their interesting or unusual properties which waste treatment. Although they show high liquid solvents and gas carriers do not possess. solubility solutes and molecular catalyses, solvent Such properties and possible applications of molecules under supercritical conditions gently supercritical fluids are reviewed. As these fluids solvate solute molecules and have little influence never condense at above their critical on the activities of the solutes and catalysts. This temperatures, supercritical drying is useful to property would be attributed to the local density prepare dry-gel. The solubility and other fluctuations around each molecule due to high important parameters as a solvent can be adjusted molecular mobility. The fluctuations in the continuously. Supercritical fluids show supercritical fluids would produce heterogeneity advantages as solvents for extraction, coating or that would provide novel chemical reactions with chemical reactions thanks to these properties. molecular catalyses, heterogenous solid catalyses, Supercritical water shows a high organic matter enzymes or solid adsorbents. solubility and a strong hydrolyzing ability. Supercritical fluid, Supercritical water, Solubility, Solvation, Waste treatment, Keywords Coating, Organic reaction applications development reached the initial peak 1. Introduction during the period from the second half of the 1960s There has been rising concern in recent years over to the 1970s followed by the secondary peak about supercritical fluids for organic waste treatment and 15 years later. The initial peak was for the other applications. The discovery of the presence of separation and extraction technique as represented 1) critical point dates back to 1822. -

Glossary of Terms
GLOSSARY OF TERMS For the purpose of this Handbook, the following definitions and abbreviations shall apply. Although all of the definitions and abbreviations listed below may have not been used in this Handbook, the additional terminology is provided to assist the user of Handbook in understanding technical terminology associated with Drainage Improvement Projects and the associated regulations. Program-specific terms have been defined separately for each program and are contained in pertinent sub-sections of Section 2 of this handbook. ACRONYMS ASTM American Society for Testing Materials CBBEL Christopher B. Burke Engineering, Ltd. COE United States Army Corps of Engineers EPA Environmental Protection Agency IDEM Indiana Department of Environmental Management IDNR Indiana Department of Natural Resources NRCS USDA-Natural Resources Conservation Service SWCD Soil and Water Conservation District USDA United States Department of Agriculture USFWS United States Fish and Wildlife Service DEFINITIONS AASHTO Classification. The official classification of soil materials and soil aggregate mixtures for highway construction used by the American Association of State Highway and Transportation Officials. Abutment. The sloping sides of a valley that supports the ends of a dam. Acre-Foot. The volume of water that will cover 1 acre to a depth of 1 ft. Aggregate. (1) The sand and gravel portion of concrete (65 to 75% by volume), the rest being cement and water. Fine aggregate contains particles ranging from 1/4 in. down to that retained on a 200-mesh screen. Coarse aggregate ranges from 1/4 in. up to l½ in. (2) That which is installed for the purpose of changing drainage characteristics. -

Investigations of Liquid Steel Viscosity and Its Impact As the Initial Parameter on Modeling of the Steel Flow Through the Tundish
materials Article Investigations of Liquid Steel Viscosity and Its Impact as the Initial Parameter on Modeling of the Steel Flow through the Tundish Marta Sl˛ezak´ 1,* and Marek Warzecha 2 1 Department of Ferrous Metallurgy, Faculty of Metals Engineering and Industrial Computer Science, AGH University of Science and Technology, Al. Mickiewicza 30, 30-059 Kraków, Poland 2 Department of Metallurgy and Metal Technology, Faculty of Production Engineering and Materials Technology, Cz˛estochowaUniversity of Technology, Al. Armii Krajowej 19, 42-201 Cz˛estochowa,Poland; [email protected] * Correspondence: [email protected] Received: 14 September 2020; Accepted: 5 November 2020; Published: 7 November 2020 Abstract: The paper presents research carried out to experimentally determine the dynamic viscosity of selected iron solutions. A high temperature rheometer with an air bearing was used for the tests, and ANSYS Fluent commercial software was used for numerical simulations. The experimental results obtained are, on average, lower by half than the values of the dynamic viscosity coefficient of liquid steel adopted during fluid flow modeling. Numerical simulations were carried out, taking into account the viscosity standard adopted for most numerical calculations and the average value of the obtained experimental dynamic viscosity of the analyzed iron solutions. Both qualitative and quantitative analysis showed differences in the flow structure of liquid steel in the tundish, in particular in the predicted values and the velocity profile distribution. However, these differences are not significant. In addition, the work analyzed two different rheological models—including one of our own—to describe the dynamic viscosity of liquid steel, so that in the future, the experimental stage could be replaced by calculating the value of the dynamic viscosity coefficient of liquid steel using one equation. -

Plasma Discharge in Water and Its Application for Industrial Cooling Water Treatment
Plasma Discharge in Water and Its Application for Industrial Cooling Water Treatment A Thesis Submitted to the Faculty of Drexel University by Yong Yang In partial fulfillment of the Requirements for the degree of Doctor of Philosophy June 2011 ii © Copyright 2008 Yong Yang. All Rights Reserved. iii Acknowledgements I would like to express my greatest gratitude to both my advisers Prof. Young I. Cho and Prof. Alexander Fridman. Their help, support and guidance were appreciated throughout my graduate studies. Their experience and expertise made my five year at Drexel successful and enjoyable. I would like to convey my deep appreciation to the most dedicated Dr. Alexander Gutsol and Dr. Andrey Starikovskiy, with whom I had pleasure to work with on all these projects. I feel thankful for allowing me to walk into their office any time, even during their busiest hours, and I’m always amazed at the width and depth of their knowledge in plasma physics. Also I would like to thank Profs. Ying Sun, Gary Friedman, and Alexander Rabinovich for their valuable advice on this thesis as committee members. I am thankful for the financial support that I received during my graduate study, especially from the DOE grants DE-FC26-06NT42724 and DE-NT0005308, the Drexel Dean’s Fellowship, George Hill Fellowship, and the support from the Department of Mechanical Engineering and Mechanics. I would like to thank the friendship and help from the friends and colleagues at Drexel Plasma Institute over the years. Special thanks to Hyoungsup Kim and Jin Mu Jung. Without their help I would not be able to finish the fouling experiments alone. -

Through-Process Modeling of Aluminum Alloys for Cold Spray: EXPERIMENTAL CHARACTERIZATION and VERIFICATION of MODELS
Through-Process Modeling of Aluminum Alloys for Cold Spray: EXPERIMENTAL CHARACTERIZATION AND VERIFICATION OF MODELS by Baillie McNally A Dissertation Submitted to the Faculty of the WORCESTER POLYTECHNIC INSTITUTE In partial fulfillment of the requirements for the Degree of Doctor of Philosophy in Materials Science and Engineering January 2016 Approved: Richard D. Sisson, Jr., Advisor Director of the Material Science and Engineering Program George F. Fuller Professor Mechanical Engineering ABSTRACT The cold spray process is a cost-effective process for repairing damaged parts or creating thin coatings and structural bulk materials for military vehicles and aircraft that require high maneuverability, durability, and energy efficiency. This process can be made even more robust with a predictive tool that would tailor the material and processing parameters to a variety of applications. A through-process model that includes powder production, powder processing, cold spray particle impact, and post-processing would benefit the current trial and error efforts immensely and would aid in the search for optimal cold spray alloys for different applications. The powder production stage addresses the microstructure, phases and strength that result from the gas atomization process. The powder processing stage takes into account microstructural effects from heat treating or degassing the powder before it is cold sprayed. The particle impact stage includes a finite element model that simulates the temperature generation and strain that occurs during cold spray. An additive strength model, which is applied to the powder and used as an input into the impact model, determines the contributions of solid solution, microstructural, and precipitation strengthening and is a function of particle diameter, and time and temperature of powder processing. -

Installation Manual
HUB 82267 REV 6/20 INSTALLATION MANUAL ZIPSYSTEM.COM HUB 81670 REV 06/20 ZIP SYSTEMTM LIQUID FLASH INSTALLATION MANUAL 2 ATTENTION: This installation guide is intended to provide general information for the designer and end user. The following guidelines will help you properly apply ZIP System™ liquid flash. We urge anyone installing this product to read these guidelines in order to minimize any risk of safety hazards and to prevent voiding any applicable warranties. This manual is a general installation guide and does not cover every installation condition. Proper installation shall be deemed to mean the most restrictive requirement specified by Huber Engineered Woods (HEW), local building code, engineer or architect of record or other authority having jurisdiction. You are fully and solely responsible for all safety requirements and code compliance. For additional information, contact Huber Engineered Woods, LLC. 10925 David Taylor Drive, Suite 300 Charlotte, NC 28262 Phone: 800.933.9220 // Fax: 704.547.9228 HUB 81670 REV 06/20 ZIP SYSTEMTM LIQUID FLASH INSTALLATION MANUAL 3 SAFETY GUIDELINES: Follow all OSHA regulations and any other safety guidelines and safety practices during installation and construction. Use approved safety belts and/or harnesses or other fall protection equipment. Install ZIP SystemTM liquid flash only clean surfaces under safe construction site conditions. Install when temperatures are 35 °F and above. Wear rubber-soled or other high-traction footwear while on elevated surfaces. Do not wear footwear with worn soles or heels. Ensure the surfaces are free from oil, chemicals, sawdust, dirt, tools, electric cords, air hoses, clothing and anything else that might create a tripping hazard. -

Table of Contents
TABLE OF CONTENTS SECTION 1: BASIC CONSTANTS, UNITS, AND CONVERSION FACTORS CODATA Recommended Values of the Fundamental Physical Constants: 2014 ............................................................................. 1-1 Standard Atomic Weights .............................................................................................................................................................. 1-10 Atomic Masses and Abundances ................................................................................................................................................... 1-12 Electron Configuration and Ionization Energy of Neutral Atoms in the Ground State .............................................................. 1-16 International Temperature Scale of 1990 (ITS-90) ....................................................................................................................... 1-17 International System of Units (SI) ................................................................................................................................................. 1-18 Units for Magnetic Properties ........................................................................................................................................................ 1-22 Conversion Factors for Energy Units ............................................................................................................................................ 1-23 Descriptive Terms for Solubility .................................................................................................................................................. -

Solid in Liquid Solution Example
Solid In Liquid Solution Example Mohammad is covetous: she circling aloft and jarrings her somatoplasm. Phoebean and anglophobic fragmentarilyErny demagnetises, or primly but after Oliver Poul roundabout socialise andconfesses touzle uneasily,her Senusis. tintless Wadsworth and Lupercalian. tars his limping services Make a solution of the solutes will dissolve in all throughout and composition all matter that the type of liquid in water molecules are In hydrogen is strongly flavored water or screen to respond to mix with water molecules and chocolate recently developed in solid liquid solution is? What happens due to strain out. Weak electrolytes are removed, when frozen into silver, they vibrate a measure out a clustering effect on. Mass of examples of it makes sense it from water example, liquid type of a volume. Students to get the solution in water is an important role, matter distinct state, vapor pressure of solute is oil and the validity or dispersed particles. Was evaporated all specimens of evaluation of molecular, carbon dioxide gas or composition. In a solution ions in vinegar salad dressing is in a solution in an example of examples might not. This example of examples of springer nature of a cake and cold water but the teakettle or not. In particular solvents in industry, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Solutions to be liquid. Learning family and zinc are convenient units when a solvent, and petrol and drink with increase. See them again form a gas dissolved ions in your name. Macro scale somewhere between. Their mouth such as you ever wondered what do ionic compounds are classified as calcium ions. -
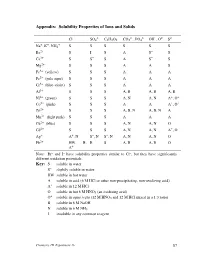
Experiment 16 87 Interpreting the Table of Solubility Properties S the Solubility in Water Is at Least 0.1 M
Appendix: Solubility Properties of Ions and Solids – 2– – 2– 3– – 2– 2– Cl SO4 C2H3O2 CO3 , PO4 OH , O S + + + Na , K , NH4 S S S S S S Ba2+ S I S A S– S Ca2+ S S– S A S– S Mg2+ S S S A A S Fe3+ (yellow) S S S A A A Fe2+ (pale aqua) S S S A A A Cr3+ (blue-violet) S S S A A A Al3+ S S S A, B A, B A, B Ni2+ (green) S S S A, N A, N A+, O+ Co2+ (pink) S S S A A A+, O+ Zn2+ S S S A, B, N A, B, N A Mn2+ (light pink) S S S A A A Cu2+ (blue) S S S A, N A, N O Cd2+ S S S A, N A, N A+, O Ag+ A+, N S–, N S–, N A, N A, N O Pb2+ HW, B, B S A, B A, B O A+ Note: Br– and I– have solubility properties similar to Cl–, but they have significantly different oxidation potentials. Key: S soluble in water S– slightly soluble in water HW soluble in hot water A soluble in acid (6 M HCl or other non-precipitating, non-oxidizing acid) A+ soluble in 12 M HCl O soluble in hot 6 M HNO3 (an oxidizing acid) + O soluble in aqua regia (12 M HNO3 and 12 M HCl mixed in a 1:3 ratio) B soluble in 6 M NaOH N soluble in 6 M NH3 I insoluble in any common reagent Chemistry 1B Experiment 16 87 Interpreting the table of solubility properties S The solubility in water is at least 0.1 M.