Universidade Estadual De Campinas Instituto De
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Phylogeny and Classification of the Melastomataceae and Memecylaceae
Nord. J. Bot. - Section of tropical taxonomy Phylogeny and classification of the Melastomataceae and Memecy laceae Susanne S. Renner Renner, S. S. 1993. Phylogeny and classification of the Melastomataceae and Memecy- laceae. - Nord. J. Bot. 13: 519-540. Copenhagen. ISSN 0107-055X. A systematic analysis of the Melastomataceae, a pantropical family of about 4200- 4500 species in c. 166 genera, and their traditional allies, the Memecylaceae, with c. 430 species in six genera, suggests a phylogeny in which there are two major lineages in the Melastomataceae and a clearly distinct Memecylaceae. Melastomataceae have close affinities with Crypteroniaceae and Lythraceae, while Memecylaceae seem closer to Myrtaceae, all of which were considered as possible outgroups, but sister group relationships in this plexus could not be resolved. Based on an analysis of all morph- ological and anatomical characters useful for higher level grouping in the Melastoma- taceae and Memecylaceae a cladistic analysis of the evolutionary relationships of the tribes of the Melastomataceae was performed, employing part of the ingroup as outgroup. Using 7 of the 21 characters scored for all genera, the maximum parsimony program PAUP in an exhaustive search found four 8-step trees with a consistency index of 0.86. Because of the limited number of characters used and the uncertain monophyly of some of the tribes, however, all presented phylogenetic hypotheses are weak. A synapomorphy of the Memecylaceae is the presence of a dorsal terpenoid-producing connective gland, a synapomorphy of the Melastomataceae is the perfectly acrodro- mous leaf venation. Within the Melastomataceae, a basal monophyletic group consists of the Kibessioideae (Prernandra) characterized by fiber tracheids, radially and axially included phloem, and median-parietal placentation (placentas along the mid-veins of the locule walls). -

Systematics and Relationships of Tryssophyton (Melastomataceae
A peer-reviewed open-access journal PhytoKeys 136: 1–21 (2019)Systematics and relationships of Tryssophyton (Melastomataceae) 1 doi: 10.3897/phytokeys.136.38558 RESEARCH ARTICLE http://phytokeys.pensoft.net Launched to accelerate biodiversity research Systematics and relationships of Tryssophyton (Melastomataceae), with a second species from the Pakaraima Mountains of Guyana Kenneth J. Wurdack1, Fabián A. Michelangeli2 1 Department of Botany, MRC-166 National Museum of Natural History, Smithsonian Institution, P.O. Box 37012, Washington, DC 20013-7012, USA 2 The New York Botanical Garden, 2900 Southern Blvd., Bronx, NY 10458, USA Corresponding author: Kenneth J. Wurdack ([email protected]) Academic editor: Ricardo Kriebel | Received 25 July 2019 | Accepted 30 October 2019 | Published 10 December 2019 Citation: Wurdack KJ, Michelangeli FA (2019) Systematics and relationships of Tryssophyton (Melastomataceae), with a second species from the Pakaraima Mountains of Guyana. PhytoKeys 136: 1–21. https://doi.org/10.3897/ phytokeys.136.38558 Abstract The systematics of Tryssophyton, herbs endemic to the Pakaraima Mountains of western Guyana, is re- viewed and Tryssophyton quadrifolius K.Wurdack & Michelang., sp. nov. from the summit of Kamakusa Mountain is described as the second species in the genus. The new species is distinguished from its closest relative, Tryssophyton merumense, by striking vegetative differences, including number of leaves per stem and leaf architecture. A phylogenetic analysis of sequence data from three plastid loci and Melastomata- ceae-wide taxon sampling is presented. The two species of Tryssophyton are recovered as monophyletic and associated with mostly Old World tribe Sonerileae. Fruit, seed and leaf morphology are described for the first time, biogeography is discussed and both species are illustrated. -

ABSTRACTS 117 Systematics Section, BSA / ASPT / IOPB
Systematics Section, BSA / ASPT / IOPB 466 HARDY, CHRISTOPHER R.1,2*, JERROLD I DAVIS1, breeding system. This effectively reproductively isolates the species. ROBERT B. FADEN3, AND DENNIS W. STEVENSON1,2 Previous studies have provided extensive genetic, phylogenetic and 1Bailey Hortorium, Cornell University, Ithaca, NY 14853; 2New York natural selection data which allow for a rare opportunity to now Botanical Garden, Bronx, NY 10458; 3Dept. of Botany, National study and interpret ontogenetic changes as sources of evolutionary Museum of Natural History, Smithsonian Institution, Washington, novelties in floral form. Three populations of M. cardinalis and four DC 20560 populations of M. lewisii (representing both described races) were studied from initiation of floral apex to anthesis using SEM and light Phylogenetics of Cochliostema, Geogenanthus, and microscopy. Allometric analyses were conducted on data derived an undescribed genus (Commelinaceae) using from floral organs. Sympatric populations of the species from morphology and DNA sequence data from 26S, 5S- Yosemite National Park were compared. Calyces of M. lewisii initi- NTS, rbcL, and trnL-F loci ate later than those of M. cardinalis relative to the inner whorls, and sepals are taller and more acute. Relative times of initiation of phylogenetic study was conducted on a group of three small petals, sepals and pistil are similar in both species. Petal shapes dif- genera of neotropical Commelinaceae that exhibit a variety fer between species throughout development. Corolla aperture of unusual floral morphologies and habits. Morphological A shape becomes dorso-ventrally narrow during development of M. characters and DNA sequence data from plastid (rbcL, trnL-F) and lewisii, and laterally narrow in M. -

Flora Da Serra Do Cipó, Minas Gerais: Microlicieae (Melastomataceae)1
29 FLORA DA SERRA DO CIPÓ, MINAS GERAIS: MICROLICIEAE (MELASTOMATACEAE)1 RICARDO PACIFICO & KARINA FIDANZA Departamento de Biologia, Universidade Estadual de Maringá, Av. Colombo, 5790, Jardim Universitário, 87020-900 - Maringá, PR, Brasil, [email protected]. Abstract – [Flora of the Serra do Cipó, Minas Gerais: Microlicieae (Melastomataceae)]. The study of the tribe Microlicieae (Melastomataceae) is part of the project “Flora da Serra do Cipó”, Minas Gerais, Brazil. According to the most recent delimitation, Microliceae comprises the genera Chaetostoma, Lavoisiera, Microlicia, Poteranthera, Rhynchanthera, Stenodon and Trembleya. In the Serra do Cipó, five genera and 69 species were recognized. The richest genera are Microlicia (42 spp.), Lavoisiera (18 spp.) e Trembleya (7 spp.), while Chaetostoma and Rhynchanthera are represented by only one species. Key to the genera and species, descriptions, color plates, and comments on the geographic distribution, phenology and variability are presented. Additionally, Microlicia damazioi Brade is proposed as a new synonym under Microlicia cordata Chamisso. Key words: campo rupestre, Espinhaço Range, eudicots, Myrtales, rosids. Resumo – [Flora da Serra do Cipó, Minas Gerais: Microlicieae (Melastomataceae)]. O estudo da tribo Microlicieae (Melastomataceae) é parte do levantamento da “Flora da Serra do Cipó”, Minas Gerais, Brasil. Em sua delimitação mais recente, Microlicieae engloba os gêneros Chaetostoma, Lavoisiera, Microlicia, Poteranthera, Rhynchanthera, Stenodon e Trembleya. Para a Serra do Cipó foram registrados cinco gêneros e 69 espécies. Os gêneros mais diversos foram Microlicia (42 spp.), Lavoisiera (18 spp.) e Trembleya (7 spp.), enquanto Chaetostoma e Rhynchanthera foram representados por apenas uma espécie. São apresentadas chaves para os gêneros e espécies, descrições, ilustrações e pranchas fotográficas das espécies de Microlicieae, além de comentários sobre sua distribuição geográfica, fenologia e variabilidade morfológica. -

Plant Structure in the Brazilian Neotropical Savannah Species
Chapter 16 Plant Structure in the Brazilian Neotropical Savannah Species Suzane Margaret Fank-de-Carvalho, Nádia Sílvia Somavilla, Maria Salete Marchioretto and Sônia Nair Báo Additional information is available at the end of the chapter http://dx.doi.org/10.5772/59066 1. Introduction This chapter presents a review of some important literature linking plant structure with function and/or as response to the environment in Brazilian neotropical savannah species, exemplifying mostly with Amaranthaceae and Melastomataceae and emphasizing the environment potential role in the development of such a structure. Brazil is recognized as the 17th country in megadiversity of plants, with 17,630 endemic species among a total of 31,162 Angiosperms [1]. The focus in the Brazilian Cerrado Biome (Brazilian Neotropical Savannah) species is justified because this Biome is recognized as a World Priority Hotspot for Conservation, with more than 7,000 plant species and around 4,400 endemic plants [2-3]. The Brazilian Cerrado Biome is a tropical savannah-like ecosystem that occupies about 2 millions of km² (from 3-24° Latitude S and from 41-43° Longitude W), with a hot, semi-humid seasonal climate formed by a dry winter (from May to September) and a rainy summer (from October to April) [4-8]. Cerrado has a large variety of landscapes, from tall savannah woodland to low open grassland with no woody plants and wetlands, as palm swamps, supporting the richest flora among the world’s savannahs-more than 7,000 native species of vascular plants- with high degree of endemism [3, 6]. The “cerrado” word is used to the typical vegetation, with grasses, herbs and 30-40% of woody plants [9-10] where trees and bushes display contorted trunk and branches with thick and fire-resistant bark, shiny coriaceous leaves and are usually recovered with dense indumentum [10]. -

2002 12 the Cerrados of Brazil.Pdf
00 oliveira fm 7/31/02 8:11 AM Page i The Cerrados of Brazil 00 oliveira fm 7/31/02 8:11 AM Page ii 00 oliveira fm 7/31/02 8:11 AM Page iii The Cerrados of Brazil Ecology and Natural History of a Neotropical Savanna Editors Paulo S. Oliveira Robert J. Marquis Columbia University Press New York 00 oliveira fm 7/31/02 8:11 AM Page iv Columbia University Press Publishers Since 1893 New York Chichester, West Sussex © 2002 Columbia University Press All rights reserved Library of Congress Cataloging-in-Publication Data The cerrados of Brazil : ecology and natural history of a neotropical savanna / Paulo S. Oliveira and Robert J. Marquis. p. cm. Includes bibliographical references. ISBN 0-231-12042-7 (cloth : alk. paper)—ISBN 0-231-12043-5 (pbk. : alk. paper) 1. Cerrado ecology—Brazil. I. Oliveira, Paulo S., 1957– II. Marquis, Robert J., 1953– QH117 .C52 2002 577.4'8'0981—dc21 2002022739 Columbia University Press books are printed on permanent and durable acid-free paper. Printed in the United States of America c 10 9 8 7 6 5 4 3 2 1 p 10 9 8 7 6 5 4 3 2 1 00 oliveira fm 7/31/02 8:11 AM Page v Contents Preface vii 1 Introduction: Development of Research in the Cerrados 1 Paulo S. Oliveira and Robert J. Marquis I Historical Framework and the Abiotic Environment 2 Relation of Soils and Geomorphic Surfaces in the Brazilian Cerrado 13 Paulo E. F. Motta, Nilton Curi, and Donald P. -

How Many Vascular Plant Species Are There in a Local Hotspot of Biodiversity in Southeastern Brazil?
Neotropical Biology and Conservation 8(3):132-142, september-december 2013 © 2013 by Unisinos - doi: 10.4013/nbc.2013.83.03 How many vascular plant species are there in a local hotspot of biodiversity in Southeastern Brazil? Quantas espécies de plantas vasculares existem em um hotspot local de biodiversidade no sudeste do Brasil? Markus Gastauer1 [email protected] Abstract Scientific information about the distribution of species richness and diversity is neces- João Augusto Alves Meira Neto2* sary for full comprehension of our evolutionary heritage forming a powerful tool for the [email protected] development of nature conservation strategies. The aim of this article was to estimate the vascular plant species richness of the campos rupestres from the Itacolomi State Park (ISP) in order to verify the park´s classification as a local hotspot of biodiversity and to outline the status quo of knowledge about biodiversity in the region. For that, the species richness of two phytosociological surveys of 0.15 ha each were extrapolated using (a) the species-area relationship fitted by the power and the logarithmic model as well as (b) the taxon ratio model. The taxon ratio model estimates total vascular plant species rich- ness to 1109 species using seven different taxa. Extrapolations of different fittings of the species-area relationships calculate the complete park’s richness to values between 241 and 386 (logarithmic model), and 3346 to 10421 (power model). These extrapolations are far beyond realistic: the logarithmic model underestimates the park´s species richness, because more than 520 vascular plant species have already been registered in the park. -
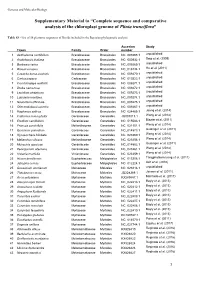
Complete Sequence and Comparative Analysis of the Chloroplast Genome of Plinia Trunciflora”
Genetics and Molecular Biology Supplementary Material to “Complete sequence and comparative analysis of the chloroplast genome of Plinia trunciflora” Table S3 - List of 56 plastome sequences of Rosids included in the Bayesian phylogenetic analysis. Accesion Study Taxon Family Order number 1 Aethionema cordifolium Brassicaceae Brassicales NC_009265.1 unpublished 2 Arabidopsis thaliana Brassicaceae Brassicales NC_000932.1 Sato et al. (1999) 3 Barbarea verna Brassicaceae Brassicales NC_009269.1 unpublished 4 Brassica napus Brassicaceae Brassicales NC_016734.1 Hu et al. (2011) 5 Capsella bursa-pastoris Brassicaceae Brassicales NC_009270.1 unpublished 6 Carica papaya Caricaceae Brassicales NC_010323.1 unpublished 7 Crucihimalaya wallichii Brassicaceae Brassicales NC_009271.1 unpublished 8 Draba nemorosa Brassicaceae Brassicales NC_009272.1 unpublished 9 Lepidium virginicum Brassicaceae Brassicales NC_009273.1 unpublished 10 Lobularia maritima Brassicaceae Brassicales NC_009274.1 unpublished 11 Nasturtium officinale Brassicaceae Brassicales NC_009275.1 unpublished 12 Olimarabidopsis pumila Brassicaceae Brassicales NC_009267.1 unpublished 13 Raphanus sativus Brassicaceae Brassicales NC_024469.1 Jeong et al. (2014) 14 California macrophylla Geraniaceae Geraniales JQ031013.1 Weng et al. (2014) 15 Erodium carvifolium Geraniaceae Geraniales NC_015083.1 Blazier et al. (2011) 16 Francoa sonchifolia Melianthaceae Geraniales NC_021101.1 Weng et al. (2014) 17 Geranium palmatum Geraniaceae Geraniales NC_014573.1 Guisinger et al. (2011) 18 Hypseocharis bilobate Geraniaceae Geraniales NC_023260.1 Weng et al. (2014) 19 Melianthus villosus Melianthaceae Geraniales NC_023256.1 Weng et al. (2014) 20 Monsonia speciose Geraniaceae Geraniales NC_014582.1 Guisinger et al. (2011) 21 Pelargonium alternans Geraniaceae Geraniales NC_023261.1 Weng et al. (2014) 22 Viviania marifolia Vivianiaceae Geraniales NC_023259.1 Weng et al. (2014) 23 Hevea brasiliensis Euphorbiaceae Malpighiales NC_015308.1 Tangphatsornruang et al. -

Molecular Phylogenetics of Melastomataceae and Memecylaceae: Implications for Character Evolution1
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Open Access LMU American Journal of Botany 88(3): 486±498. 2001. MOLECULAR PHYLOGENETICS OF MELASTOMATACEAE AND MEMECYLACEAE: IMPLICATIONS FOR CHARACTER EVOLUTION1 G. CLAUSING2,4 AND S. S. RENNER3,4 2Institut fuÈr Spezielle Botanik, UniversitaÈt Mainz, D-55099 Mainz, Germany; 3Department of Biology, University of Missouri-St. Louis, 8001 Natural Bridge Rd., St. Louis, Missouri 63121 USA; and The Missouri Botanical Garden, St. Louis, Missouri 63166 USA Melastomataceae are among the most abundant and diversi®ed groups of plants throughout the tropics, but their intrafamily rela- tionships and morphological evolution are poorly understood. Here we report the results of parsimony and maximum likelihood (ML) analyses of cpDNA sequences from the rbcL and ndhF genes and the rpl16 intron, generated for eight outgroups (Crypteroniaceae, Alzateaceae, Rhynchocalycaceae, Oliniaceae, Penaeaceae, Myrtaceae, and Onagraceae) and 54 species of melastomes. The sample represents 42 of the family's currently recognized ;150 genera, the 13 traditional tribes, and the three subfamilies, Astronioideae, Melastomatoideae, and Memecyloideae (5 Memecylaceae DC.). Parsimony and ML yield congruent topologies that place Memecy- laceae as sister to Melastomataceae. Pternandra, a Southeast Asian genus of 15 species of which ®ve were sampled, is the ®rst- branching Melastomataceae. This placement has low bootstrap support (72%), but agrees with morphological treatments that placed Pternandra in Melastomatacaeae because of its acrodromal leaf venation, usually ranked as a tribe or subfamily. The interxylary phloem islands found in Memecylaceae and Pternandra, but not most other Melastomataceae, likely evolved in parallel because Pternandra resembles Melastomataceae in its other wood characters. -

1 Updates Required to Plant Systematics: A
Updates Required to Plant Systematics: A Phylogenetic Approach, Third Edition, as a Result of Recent Publications (Updated June 13, 2014) As necessitated by recent publications, updates to the Third Edition of our textbook will be provided in this document. It is hoped that this list will facilitate the efficient incorporation new systematic information into systematic courses in which our textbook is used. Plant systematics is a dynamic field, and new information on phylogenetic relationships is constantly being published. Thus, it is not surprising that even introductory texts require constant modification in order to stay current. The updates are organized by chapter and page number. Some require only minor changes, as indicated below, while others will require more extensive modifications of the wording in the text or figures, and in such cases we have presented here only a summary of the major points. The eventual fourth edition will, of course, contain many organizational changes not treated below. Page iv: Meriania hernandii Meriania hernandoi Chapter 1. Page 12, in Literature Cited, replace “Stuessy, T. F. 1990” with “Stuessy, T. F. 2009,” which is the second edition of this book. Stuessy, T. F. 2009. Plant taxonomy: The systematic evaluation of comparative data. 2nd ed. Columbia University Press, New York. Chapter 2. Page 37, column 1, line 5: Stuessy 1983, 1990;… Stuessy 1983, 2009; … And in Literature Cited, replace “Stuessy 1990” with: Stuessy, T. F. 2009. Plant taxonomy: The systematic evaluation of comparative data. 2nd ed. Columbia University Press, New York. Chapter 4. Page 58, column 1, line 5: and Dilcher 1974). …, Dilcher 1974, and Ellis et al. -

Botany News. 2. May 20, 2020 California Academy of Sciences
Botany News. 2. May 20, 2020 California Academy of Sciences Tristemma mauritianum (A Madagascar Princess Flower) F. Almeda Hello to our volunteers, We celebrated a milestone this month. It has been 15 years since Deb Trock (Director of Collections and Botany Collections Manager) joined the Academy! We held a surprise party for her online--she was expecting a boring meeting and instead she was greeted with home-made signs saying congratulations (one of the fancier ones is below), many smiling faces, and speeches recognizing her contributions to the Academy collections and to the staff. We would have preferred sharing this special moment with her in-person, but we are adapting and enjoying the time that we can spend together virtually. We hope you enjoy our second newsletter, and we hope you are all keeping well and busy. With our best wishes from the Botany Department A Walk Through the Orchids in San Francisco Tom Daniel During my walk home from the Academy through four or five different neighborhoods on the west side of San Francisco, I see several different large-flowered and brightly colored orchids that residents have in their yards, porches, and windows. For the past few years, I have been growing a beautiful orchid in a bark-filled pot in my backyard. Our department’s research associate and orchid specialist, De Mally, gave Mary and me a cluster of pseudobulbs of Maxillaria soconuscana (recently transferred to a different genus, as Psittacoglossum soconuscanum), a native of Chiapas, Mexico that former botany curator Dennis Breedlove collected in 1986, and which she and Dennis described as new to science in 1989. -

The Systematic Distribution of Vascular Epiphytes: an Update
Selbyana 9: 2-22 THE SYSTEMATIC DISTRIBUTION OF VASCULAR EPIPHYTES: AN UPDATE w. JOHN KREss Marie Selby Botanical Gardens, 811 South Palm Avenue, Sarasota, Florida 33577 ABSTRACT. The list of vascular plant families and genera containing epiphytic species published by Madison (1977) is updated here. Ten percent (23,456 species) of all vascular plant species are epiphytes. Seven percent (876) of the genera, 19 percent (84) of the families, 45 percent (44) of the orders and 75 percent (6) of the classes contain epiphytes. Twenty-three families contain over 50 epiphytic species each. The Orchidaceae is the largest family of epiphytes, containing 440 epiphytic genera and 13,951 epiphytic species. Forty-three genera of vascular plants each contain over 100 epiphytic species. In 1977 Madison published a list of the vas those plants that normally spend their entire life cular plant families and genera that contain epi cycle perched on another plant and receive all phytic species. Madison compiled this list from mineral nutrients from non-terrestrial sources. literature reports, consultation with taxonomic Hemi~epiphytes normally spend only part oftheir specialists, and a survey of herbarium material. life cycle perched on another plant and thus some He reported that 65 families contain 850 genera mineral nutrients are received from terrestrial and 28,200 species of epiphytes. His total ac sources. Hemi-epiphytes either begin their life counted for about ten percent of all species of cycle as epiphytes and eventually send roots and vascular plants. shoots to the ground (primary hemi-epiphytes), Madison's list was the most comprehensive or begin as terrestrially established seedlings that compilation of vascular epiphytes since the works secondarily become epiphytic by severing all of Schimper (1888) and Richards (1952) upon connections with the ground (secondary hemi which it was partly based.