Smithsonian Miscellaneous Collections
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Reproducibility of Silver-Silver Halide Electrodes
U. S. DEPARTMENT OF COMMERCE NATIONAL BUREAU OF STANDARDS RESEARCH PAPER RP1183 Part of Journal of Research of the National Bureau of Standards, Volume 22, March 1939 REPRODUCIBILITY OF SILVER.SILVER HALIDE ELECTRODES 1 By John Keenan Taylor and Edgar Reynolds Smith ABSTRACT Tests of the reproducibility in potential of the electrolytic, thermal-electrolytic, and thermal types of silver-silver chloride, silver-silver bromide, and silver-silver iodide electrodes, in both acid and neutral solutions, are reported. All of these silver-silver halide electrodes show an aging effect, such that freshly prepared electrodes behave as cathodes towards electrodes previously aged in the solution. They are not affected in potential by exposure to light, but the presence of oxygen disturbs the potentials of the silver-silver chloride and silver-silver bromide elec trodes in acid solutions, and of the silver-silver iodide electrodes in both acid and neutral solutions. Except in the case of the silver-silver iodide electrodes, of which the thermal-electrolytic type seems more reliable than the electrolytic or the thermal type, the equilibrium potential is independent of the type, within about 0.02 mv. CONTENTS Page I. Introduetion_ __ _ _ _ __ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ __ _ _ _ _ _ __ _ _ _ _ _ _ _ _ _ _ _ 307 II. Apparatus and materials_ _ _ _ _ _ _ _ _ _ _ _ _ __ __ _ _ ___ _ _ ____ _ _ _ _ ___ _ _ _ _ 308 III. -

1.Brethericks1 51 1..51
0001. Silver [7440-22-4] Ag Ag Acetylenic compounds MRH Acetylene 8.70/99þ See ACETYLENIC COMPOUNDS Aziridine See Aziridine: Silver Bromine azide See Bromine azide 3-Bromopropyne See 3-Bromopropyne: Metals Carboxylic acids Koffolt, J. H., private comm., 1965 Silver is incompatible with oxalic or tartaric acids, since the silver salts decompose on heating. Silver oxalate explodes at 140C, and silver tartrate loses carbon dioxide. See other METAL OXALATES Chlorine trifluoride MRH 1.42/36 See Chlorine trifluoride: Metals Copper, Ethylene glycol See Ethylene glycol: Silvered copper wire Electrolytes, Zinc Britz, W. K. et al., Chem. Abs., 1975, 83, 150293 Causes of spontaneous combustion and other hazards of silver—zinc batteries were investigated. Ethanol, Nitric acid Luchs, J. K., Photog. Sci. Eng., 1966, 10, 334 Action of silver on nitric acid in presence of ethanol may form the readily detonable silver fulminate. See Nitric acid: Alcohols See also SILVER-CONTAINING EXPLOSIVES Ethyl hydroperoxide See Ethyl hydroperoxide: Silver Ethylene oxide MRH 3.72/99þ See Ethylene oxide: Reference 4 Hydrogen peroxide MRH 1.59/99þ See Hydrogen peroxide: Metals Iodoform Grignard, 1935, Vol. 3, 320 In contact with finely divided (reduced) silver, incandescence occurs. 1 Other reactants Yoshida, 1980, 103 MRH values for 7 combinations, largely with catalytically susceptible materials, are given. Ozonides See OZONIDES Peroxomonosulfuric acid See Peroxomonosulfuric acid: Catalysts Peroxyformic acid MRH 5.69/100 See Peroxyformic acid: Metals See other METALS 0002. Silver—aluminium alloy [11144-29-9] AgÀAl Ag Al 1. Popov, E. I. et al., Chem. Abs., 1977, 87, 205143 2. Popov, E. I. et al., Chem. -

Chemical Names and CAS Numbers Final
Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number C3H8O 1‐propanol C4H7BrO2 2‐bromobutyric acid 80‐58‐0 GeH3COOH 2‐germaacetic acid C4H10 2‐methylpropane 75‐28‐5 C3H8O 2‐propanol 67‐63‐0 C6H10O3 4‐acetylbutyric acid 448671 C4H7BrO2 4‐bromobutyric acid 2623‐87‐2 CH3CHO acetaldehyde CH3CONH2 acetamide C8H9NO2 acetaminophen 103‐90‐2 − C2H3O2 acetate ion − CH3COO acetate ion C2H4O2 acetic acid 64‐19‐7 CH3COOH acetic acid (CH3)2CO acetone CH3COCl acetyl chloride C2H2 acetylene 74‐86‐2 HCCH acetylene C9H8O4 acetylsalicylic acid 50‐78‐2 H2C(CH)CN acrylonitrile C3H7NO2 Ala C3H7NO2 alanine 56‐41‐7 NaAlSi3O3 albite AlSb aluminium antimonide 25152‐52‐7 AlAs aluminium arsenide 22831‐42‐1 AlBO2 aluminium borate 61279‐70‐7 AlBO aluminium boron oxide 12041‐48‐4 AlBr3 aluminium bromide 7727‐15‐3 AlBr3•6H2O aluminium bromide hexahydrate 2149397 AlCl4Cs aluminium caesium tetrachloride 17992‐03‐9 AlCl3 aluminium chloride (anhydrous) 7446‐70‐0 AlCl3•6H2O aluminium chloride hexahydrate 7784‐13‐6 AlClO aluminium chloride oxide 13596‐11‐7 AlB2 aluminium diboride 12041‐50‐8 AlF2 aluminium difluoride 13569‐23‐8 AlF2O aluminium difluoride oxide 38344‐66‐0 AlB12 aluminium dodecaboride 12041‐54‐2 Al2F6 aluminium fluoride 17949‐86‐9 AlF3 aluminium fluoride 7784‐18‐1 Al(CHO2)3 aluminium formate 7360‐53‐4 1 of 75 Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number Al(OH)3 aluminium hydroxide 21645‐51‐2 Al2I6 aluminium iodide 18898‐35‐6 AlI3 aluminium iodide 7784‐23‐8 AlBr aluminium monobromide 22359‐97‐3 AlCl aluminium monochloride -

Standard X-Ray Diffraction Powder Patterns NATIONAL BUREAU of STANDARDS
NBS MONOGRAPH 25—SECTION 1 9 CO Q U.S. DEPARTMENT OF COMMERCE/National Bureau of Standards Standard X-ray Diffraction Powder Patterns NATIONAL BUREAU OF STANDARDS The National Bureau of Standards' was established by an act of Congress on March 3, 1901. The Bureau's overall goal is to strengthen and advance the Nation's science and technology and facilitate their effective application for public benefit. To this end, the Bureau conducts research and provides: (1) a basis for the Nation's physical measurement system, (2) scientific and technological services for industry and government, (3) a technical basis for equity in trade, and (4) technical services to promote public safety. The Bureau's technical work is per- formed by the National Measurement Laboratory, the National Engineering Laboratory, and the Institute for Computer Sciences and Technology. THE NATIONAL MEASUREMENT LABORATORY provides the national system of physical and chemical and materials measurement; coordinates the system with measurement systems of other nations and furnishes essentia! services leading to accurate and uniform physical and chemical measurement throughout the Nation's scientific community, industry, and commerce; conducts materials research leading to improved methods of measurement, standards, and data on the properties of materials needed by industry, commerce, educational institutions, and Government; provides advisory and research services to other Government agencies; develops, produces, and distributes Standard Reference Materials; and provides calibration -

SILVER COMPOUNDS 1. Introduction Silver, a White, Lustrous Metal
SILVER COMPOUNDS 1. Introduction Silver, a white, lustrous metal, slightly less malleable and ductile than gold (see GOLD AND GOLD COMPOUNDS), has high thermal and electrical conductivity (see SIL- VER AND SILVER ALLOYS). Most silver compounds are made from silver nitrate [7761- 88-8], AgNO3, which is prepared from silver metal. Some silver metal is found in Nature, frequently alloyed with other metals such as copper, lead, or gold. Naturally occurring silver compounds, however, are the primary sources of silver in the environment. The most abundant naturally occurring silver compound is silver sulfide [21548- 73-2] (argentite), Ag2S, found alone and combined with iron, copper, and lead sulfides. Other naturally occur- ring silver compounds are silver sulfonantimonite [15983-65-0] (pyrargyrite), Ag3SbS3, silver arsenite [15122-57-3] (proustite), Ag3AsS3, silver selenide [1302-09-6], and silver telluride [12653-91-7]. Silver chloride (chlorargyrite) and silver iodide (iodargyrite) have also been found in substantial quantities in the western United States. Silver is a soft metal that preferentially forms lower oxidation states (þ1, þ2) and binds preferentially with sulfur (1). Studies show that silver levels in uncontaminated fine-grain sediments are typically similar to those for average crustal abundance, 0.05mg/g concentration of silver in fresh lake and river waters is typically<0.2mg/L, while those in contaminated estuary and harbor waters may be upward of from 5.1 to 33.9mg/g (2) produces a linear co-ordination of connecting bonds (3). It has one of the highest electronegativities resulting in strong covalent bonds. Its oxides are weakly held and are dissociate at about 2008C. -
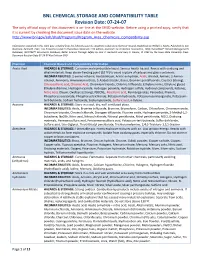
BNL CHEMICAL STORAGE and COMPATIBILITY TABLE Revision Date: 07-24-07 the Only Official Copy of This Document Is On-Line at the SHSD Website
BNL CHEMICAL STORAGE AND COMPATIBILITY TABLE Revision Date: 07-24-07 The only official copy of this document is on-line at the SHSD website. Before using a printed copy, verify that it is current by checking the document issue date on the website. http://www.bnl.gov/esh/shsd/Programs/Program_Area_Chemicals_Compatibility.asp Information contained in this table was compiled from the following sources: Academic Laboratory Chemical Hazards Guidebook by William J. Mahn, Published by Van Nostrand, Reinhold, 1991; Fire Protection Guide to Hazardous Materials 11th edition, National Fire Protection Association, 1994; Hazardtext® Hazard Managements Database; INFOTEXT® Documents Database; Better Science Through Safety by Jack A. Gerlovich and Gary E. Downs, © 1981 by the Iowa State University Press. Document Revision Date 07-24-07 Ken Erickson CHO Chemical Chemical Hazard and Compatibility Information Acetic Acid HAZARDS & STORAGE: Corrosive and combustible liquid. Serious health hazard. Reacts with oxidizing and alkali materials. Keep above freezing point (62 ºF) to avoid rupture of carboys and glass containers. INCOMPATIBILITIES: 2-amino-ethanol, Acetaldehyde, Acetic anhydride, Acids, Alcohol, Amines, 2-Amino- ethanol, Ammonia, Ammonium nitrate, 5-Azidotetrazole, Bases, Bromine pentafluoride, Caustics (strong), Chlorosulfonic acid, Chromic Acid, Chromium trioxide, Chlorine trifluoride, Ethylene imine, Ethylene glycol, Ethylene diamine, Hydrogen cyanide, Hydrogen peroxide, Hydrogen sulfide, Hydroxyl compounds, Ketones, Nitric Acid, Oleum, Oxidizers -
Standard X-Ray Diffraction Powder Patterns
E^l Admin. NBS MONOGRAPH 25—SECTION 5 Refecii^M not to be ^ferlrom the library. Standard X-ray Diffraction Powder Patterns ^\ / U.S. DEPARTMENT OF COMMERCE S NATIONAL BUREAU OF STANDARDS THE NATIONAL BUREAU OF STANDARDS The National Bureau of Standards^ provides measurement and technical information services essential to the efficiency and effectiveness of the work of the Nation's scientists and engineers. The Bureau serves also as a focal point in the Federal Government for assuring maximum application of the physical and engineering sciences to the advancement of technology in industry and commerce. To accomplish this mission, the Bureau is organized into three institutes covering broad program areas of research and services: THE INSTITUTE FOR BASIC STANDARDS . provides the central basis within the United States for a complete and consistent system of physical measurements, coordinates that system with the measurement systems of other nations, and furnishes essential services leading to accurate and uniform physical measurements throughout the Nation's scientific community, industry, and commerce. This Institute comprises a series of divisions, each serving a classical subject matter area: —Applied Mathematics—Electricity—Metrology—Mechanics—Heat—Atomic Physics—Physical Chemistry—Radiation Physics— -Laboratory Astrophysics^—Radio Standards Laboratory,^ which includes Radio Standards Physics and Radio Standards Engineering—Office of Standard Refer- ence Data. THE INSTITUTE FOR MATERIALS RESEARCH . conducts materials research and provides associated materials services including mainly reference materials and data on the properties of ma- terials. Beyond its direct interest to the Nation's scientists and engineers, this Institute yields services which are essential to the advancement of technology in industry and commerce. -

Standard X-Ray Diffraction Powder Patterns
7 NATL INST OF STANDARDS & TECH R.I.C. Nes AlllOD Ififib^fi PUBLICATIONS 100988698 ST5°5rv'25-17;1980 C.1 NBS-PUB-C 19 cr»T OF NBS MONOGRAPH 25-SECTION 1 V) J U.S. DEPARTMENT OF COMMERCE / National Bureau of Standards Standard X-ray Diffraction Powder Patterns . NATIONAL BUREAU OF STANDARDS The National Bureau of Standards' was established by an act ot Congress on March 3, 1901 The Bureau's overall goal is to strengthen and advance the Nation's science and technology and facilitate their effective application for public benefit. To this end, the Bureau conducts research and provides: (1) a basis for the Nation's physical measurement system, (2) scientific and technological services for industry and government, (3) a technical basis for equity in trade, and (4) technical services to promote public safety. The Bureau's technical work is per- formed by the National Measurement Laboratory, the National Engineering Laboratory, and the Institute for Computer Sciences and Technology. THE NATIONAL MEASUREMENT LABORATORY provides the national system of physical and chemical and materials measurement; coordinates the system with measurement systems of other nations and furnishes essential services leading to accurate and uniform physical and chemical measurement throughout the Nation's scientific community, industry, and commerce; conducts materials research leading to improved methods ol measurement, standards, and data on the properties of materials needed by industry, commerce, educational institutions, and Government; provides advisory and research services to other Government agencies; develops, produces, and distributes Standard Reference Materials; and provides calibration services. The Laboratory consists of the following centers: Absolute Physical Quantities- — Radiation Research — Thermodynamics and Molecular Science — Analytical Chemistry — Materials Science. -

Specialty Silver Compounds
Specialty Silver Compounds • Selection of silver compounds can be manufactured up to a purity of 99.999% • Batch production capability from grams to 200 Kg www.alfa.com Alfa Aesar have the capability of Specialized Silver Compounds producing batch sizes up to 200 Kg. Bulk packing sizes are customized to • Our specialized silver compounds are meet our customers’ requirements. available in catalog sizes to bulk quantities. Many silver compounds are • We can manufacture our silver compounds to specific purity on request. photosensitive. All our specialized silver compounds are packaged in lightproof • If you require a specialized silver compound containers. not listed we can discuss its manufacture. KEY FEATURES Manufacturing • A batch specific certificate of analysis is produced for each product. Alfa Aesar’s production operations offer a single source for silver salts from research to pilot plant • Each silver product batch has; scale. Full-scale production of these products is 1. Full metallic impurities typically measured available. by ICP-MS to ppm. 2. The silver content measured. Development • A selection of silver compounds can be With our extensive development capability, we manufactured up to a purity of 99.999% are able to supply advanced silver materials to meet your technical requirements. • Most other silver products are manufactured to 99.9% purity as standard. Quality Control We employ advanced quality control for both in-process and final product testing phases. The high standard of our modern quality control and assurance facilities is matched by the expertise of our experienced staff. Further details and prices for catalog quantities are available in the Alfa Aesar Catalog or via the web site www.alfa.com. -

DAP Sulphur Super
DAP Sulphur Super Ballance Agri-Nutrients Chemwatch Hazard Alert Code: 3 Chemwatch: 5174-21 Issue Date: 25/08/2021 Version No: 5.1.7.9 Print Date: 25/08/2021 Safety Data Sheet according to the Health and Safety at Work (Hazardous Substances) Regulations 2017 L.GHS.NZL.EN SECTION 1 Identification of the substance / mixture and of the company / undertaking Product Identifier Product name DAP Sulphur Super Chemical Name Not Applicable Chemical formula Not Applicable Other means of identification Not Available Relevant identified uses of the substance or mixture and uses advised against Relevant identified uses Fertiliser. Details of the supplier of the safety data sheet Registered company name Ballance Agri-Nutrients Address 161 Hewletts Rd Mount Maunganui New Zealand Telephone +64 800 222 090 Fax Not Available Website Not Available Email [email protected] Emergency telephone number Association / Organisation CHEMCALL Emergency telephone Freephone: 0800 CHEMCALL (0800 243 622) (24 Hours/ 7 Days) numbers Other emergency telephone Not Available numbers SECTION 2 Hazards identification Classification of the substance or mixture Considered a Hazardous Substance according to the criteria of the New Zealand Hazardous Substances New Organisms legislation. Not regulated for transport of Dangerous Goods. ChemWatch Hazard Ratings Min Max Flammability 0 Toxicity 1 0 = Minimum Body Contact 3 1 = Low Reactivity 0 2 = Moderate 3 = High Chronic 0 4 = Extreme Skin Corrosion/Irritation Category 2, Serious Eye Damage/Eye Irritation Category 2, Hazardous to the Aquatic Environment Long-Term Hazard Classification [1] Category 4 Legend: 1. Classified by Chemwatch; 2. Classification drawn from CCID EPA NZ; 3. -

Standard X-Ray Diffraction Powder Patterns NATIONAL BUREAU of STANDARDS
MDC UNIV. of ARIZONA MONOGRAPH 25-SECTION 18 Standard x-ray diffraction powder pattern C 13.44: 25/sec.18 \ VAU o? * 3 9001 90045 6248 U.S. DEPARTMENT OF COMMERCE/ National Bureau of Standards Standard X-ray Diffraction Powder Patterns NATIONAL BUREAU OF STANDARDS The National Bureau of Standards© was established by an act of Congress on March 3, 1901. The Bureau©s overall goal is to strengthen and advance the Nation©s science and technology and facilitate their effective application for public benefit. To this end, the Bureau conducts research and provides: (1) a basis for the Nation©s physical measurement system, (2) scientific and technological services for industry and government, (3) a technical basis for equity in trade, and (4) technical services to promote public safety. The Bureau©s technical work is per formed by the National Measurement Laboratory, the National Engineering Laboratory, and the Institute for Computer Sciences and Technology. THE NATIONAL MEASUREMENT LABORATORY provides the national system of physical and chemical and materials measurement; coordinates the system with measurement systems of other nations and furnishes essential services leading to accurate and uniform physical and chemical measurement throughout the Nation©s scientific community, industry, and commerce; conducts materials research leading to improved methods of measurement, standards, and data on the properties of materials needed by industry, commerce, educational institutions, and Government; provides advisory and research services to other Government agencies; develops, produces, and distributes Standard Reference Materials; and provides calibration services. The Laboratory consists of the following centers: Absolute Physical Quantities2 Radiation Research Thermodynamics and Molecular Science Analytical Chemistry Materials Science. -

Standard X-Ray Diffraction Powder Patterns
0 UO100 .U556 V25-18;1981 C.1 NBS-PUB-C 19 ^ ^ NBS MONOGRAPH 2b-SECTI0N 1 Standard X-ray Diffraction Powder Patterns 100 .U555 NO. 18 Sect. 18 1981 NATIONAL BUREAU OF STANDARDS The National Bureau of Standards' was established by an act ot Congress on March 3, 1901. The Bureau's overall goal is to strengthen and advance the Nation's science and technology and facilitate their effective application for public benefit. To this end, the Bureau conducts research and provides: (1) a basis for the Nation's physical measurement system, (2) scientific and technological services for industry and government, (3) a technical basis for equity in trade, and (4) technical services to promote public safety. The Bureau's technical work is per- formed by the National Measurement Laboratory, the National Engineering Laboratory, and the Institute for Computer Sciences and Technology. THE NATIONAL MEASUREMENT LABORATORY provides the national system of physical and chemical and materials measurement; coordinates the system with measurement systems of other nations and furnishes essential services leading to accurate and uniform physical and chemical measurement throughout the Nation's scientific community, industry, and commerce; conducts materials research leading to improved methods of measurement, standards, and data on the properties of materials needed by industry, commerce, educational institutions, and Government; provides advisory and research services to other Government agencies; develops, produces, and distributes Standard Reference Materials;