Geography, Assortative Mating, and the Effects of Sexual Selection on Speciation with Gene flow Maria R
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Robust Test for Assortative Mating
European Journal of Human Genetics (2000) 8, 119–124 © 2000 Macmillan Publishers Ltd All rights reserved 1018–4813/00 $15.00 y www.nature.com/ejhg ARTICLE A robust test for assortative mating Emmanuelle G´enin1, Carole Ober2, Lowell Weitkamp3 and Glenys Thomson1 1Department of Integrative Biology, University of California, Berkeley, CA, USA; 2Department of Human Genetics, University of Chicago, Chicago, IL, USA; 3Department of Psychiatry and Division of Genetics, University of Rochester Medical Center, Rochester, NY, USA Testing for random mating in human populations is difficult due to confounding factors such as ethnic preference and population stratification. With HLA, the high level of polymorphism is an additional problem since it is rare for couples to share the same haplotype. Focus on an ethnically homogeneous population, where levels of polymorphism at HLA loci are more limited, may provide the best situation in which to detect non-random mating. However, such populations are often genetic isolates where there may be inbreeding to an extent that is difficult to quantify and account for. We have developed a test for random mating at a multiallelic locus that is robust to stratification and inbreeding. This test relies on the availability of genotypic information from the parents of both spouses. The focus of the test is on families where there is allele sharing between the parents of both spouses, so that potential spouses could share an allele. Denoting the shared allele at the locus of interest by A, then under the assumption of random mating, heterozygous parents AX should transmit allele A equally as frequently as allele X to their offspring. -

And Ford, I; Ford, '953) on the Other Hand Have Put Forward a View Intermediate Between the Extreme Ones of Darwin on the One Hand and Goldschmidt on the Other
THE EVOLUTION OF MIMICRY IN THE BUTTERFLY PAPILIO DARDANUS C. A. CLARKE and P. M. SHEPPARD Departments of Medicine and Zoology, University of Liverpool Received23.V.59 1.INTRODUCTION WHENBatesputforward the mimicry hypothesis which bears his name, Darwin (1872), although accepting it, had some difficulty in explaining the evolution of the mimetic resemblance of several distinct species to one distasteful model by a series of small changes, a require- ment of his general theory of evolution. He said "it is necessary to suppose in some cases that ancient members belonging to several distinct groups, before they had diverged to their present extent, accidentally resembled a member of another and protected group in a sufficient degree to afford some slight protection; this having given the basis for the subsequent acquisition of the most perfect resemb- lance ". Punnett (1915) realised that the difficulty is even more acute when one is dealing with a polymorphic species whose forms mimic very distantly related models. Knowing that, in those butterflies which had been investigated genetically, the forms differed by single allelomorphs he concluded that the mimicry did not evolve gradually and did not confer any advantage or disadvantage to the individual. He argued that an allelomorph arises at a single step by mutation and that therefore the mimicry also arises by chance at a single step. Goldschmidt (x) although not denying that mimicry confers some advantage to its possessors also maintained that the resemblance arises fully perfected by a single mutation of a gene distinct from that producing the colour pattern in the model, but producing a similar effect in the mimic. -

Microevolution and the Genetics of Populations Microevolution Refers to Varieties Within a Given Type
Chapter 8: Evolution Lesson 8.3: Microevolution and the Genetics of Populations Microevolution refers to varieties within a given type. Change happens within a group, but the descendant is clearly of the same type as the ancestor. This might better be called variation, or adaptation, but the changes are "horizontal" in effect, not "vertical." Such changes might be accomplished by "natural selection," in which a trait within the present variety is selected as the best for a given set of conditions, or accomplished by "artificial selection," such as when dog breeders produce a new breed of dog. Lesson Objectives ● Distinguish what is microevolution and how it affects changes in populations. ● Define gene pool, and explain how to calculate allele frequencies. ● State the Hardy-Weinberg theorem ● Identify the five forces of evolution. Vocabulary ● adaptive radiation ● gene pool ● migration ● allele frequency ● genetic drift ● mutation ● artificial selection ● Hardy-Weinberg theorem ● natural selection ● directional selection ● macroevolution ● population genetics ● disruptive selection ● microevolution ● stabilizing selection ● gene flow Introduction Darwin knew that heritable variations are needed for evolution to occur. However, he knew nothing about Mendel’s laws of genetics. Mendel’s laws were rediscovered in the early 1900s. Only then could scientists fully understand the process of evolution. Microevolution is how individual traits within a population change over time. In order for a population to change, some things must be assumed to be true. In other words, there must be some sort of process happening that causes microevolution. The five ways alleles within a population change over time are natural selection, migration (gene flow), mating, mutations, or genetic drift. -

Patterns and Power of Phenotypic Selection in Nature
Articles Patterns and Power of Phenotypic Selection in Nature JOEL G. KINGSOLVER AND DAVID W. PFENNIG Phenotypic selection occurs when individuals with certain characteristics produce more surviving offspring than individuals with other characteristics. Although selection is regarded as the chief engine of evolutionary change, scientists have only recently begun to measure its action in the wild. These studies raise numerous questions: How strong is selection, and do different types of traits experience different patterns of selection? Is selection on traits that affect mating success as strong as selection on traits that affect survival? Does selection tend to favor larger body size, and, if so, what are its consequences? We explore these questions and discuss the pitfalls and future prospects of measuring selection in natural populations. Keywords: adaptive landscape, Cope’s rule, natural selection, rapid evolution, sexual selection henotypic selection occurs when individuals with selection on traits that affect survival stronger than on those Pdifferent characteristics (i.e., different phenotypes) that affect only mating success? In this article, we explore these differ in their survival, fecundity, or mating success. The idea and other questions about the patterns and power of phe- of phenotypic selection traces back to Darwin and Wallace notypic selection in nature. (1858), and selection is widely accepted as the primary cause of adaptive evolution within natural populations.Yet Darwin What is selection, and how does it work? never attempted to measure selection in nature, and in the Selection is the nonrandom differential survival or repro- century following the publication of On the Origin of Species duction of phenotypically different individuals. -

Reinforcement and the Genetics of Hybrid Incompatibilities
Genetics: Published Articles Ahead of Print, published on March 17, 2006 as 10.1534/genetics.105.048199 Reinforcement and the genetics of hybrid incompatibilities Alan R. Lemmon1 and Mark Kirkpatrick Affiliation: Section of Integrative Biology, The University of Texas at Austin, Austin, TX 78712 1 Running head: Reinforcement and hybrid incompatibility Keywords: hybridization, speciation, reinforcement, incompatibility, sex linkage, incompatibility sieve 1Correspondence: Alan R. Lemmon Section of Integrative Biology The University of Texas at Austin 1 University Station #C0930 Austin, TX 78712 USA Email: [email protected] Tel: 512-471-3760; Fax: 512-471-3878 2 ABSTRACT Recent empirical studies suggest that genes involved in speciation are often sex-linked. We derive a general analytic model of reinforcement to study the effects of sex linkage on reinforcement under three forms of selection against hybrids: one-locus, two-locus, and ecological incompatibilities. We show that the pattern of sex linkage can have a large effect on the amount of reinforcement due to hybrid incompatibility. Sex linkage of genes involved in postzygotic isolation generally increases the strength of reinforcement, but only if genes involved in prezygotic isolation are also sex-linked. We use exact simulations to test the accuracy of the approximation and find that qualitative predictions made assuming weak selection can hold when selection is strong. 3 Speciation is the evolution of prezygotic or postzygotic isolation. Postzygotic isolation is thought to evolve through the accumulation of genetic incompatibilities during allopatric separation. Prezygotic isolation can evolve through reinforcement, which is the evolution of increased prezygotic isolation as a result of selection against hybrids (Dobzhansky 1940; Blair 1955; Howard 1993). -

Arise by Chance As the Result of Mutation. They Therefore Suggest
THE EVOLUTION OF DOMINANCE UNDER DISRUPTIVE SELECTION C. A. CLARKE and P. Ni. SHEPPARD Department of Medicine and Department of Zoology, University of Liverpool Received6.iii.59 1.INTRODUCTION INa paper on the effects of disruptive selection, Mather (1955) pointed out that if there are two optimum values for a character and all others are less advantageous or disadvantageous there will be disruptive selection which can lead to the evolution of a polymorphism. Sheppard (1958) argued that where such selection is effective and the change from one optimum value to the other is switched by a single pair of allelomorphs there will be three genotypes but only two advantageous phenotypes. Consequently if dominance were absent initially it would be evolved as a result of the disruptive selection, the heterozygote and one of the homozygotes both coming to resemble one of the two optimum phenotypes (see Ford, 1955, on Tripharna comes). Thoday (1959) has shown by means of an artificial selection experiment that, even when a character is, at the beginning, controlled polygenically (sternopleural chaeta-number in Drosophila) and there is 50 per cent. gene exchange between the "high" and "low" selected sub-popu- lations, a polymorphism can evolve. The most fully understood examples of disruptive selection (other than sex) are provided by instances of Batesian Mimicry, where there are a number of distinct warningly coloured species, acting as models, which are mimicked by the polymorphic forms of a single more edible species. Fisher and Ford (see Ford, 1953) have argued that a suffi- ciently good resemblance between mimic and model is not likely to arise by chance as the result of mutation. -

Sex Differences in the Recombination Landscape*
vol. 195, no. 2 the american naturalist february 2020 Symposium Sex Differences in the Recombination Landscape* Jason M. Sardell† and Mark Kirkpatrick Department of Integrative Biology, University of Texas at Austin, Austin, Texas 78712 Submitted May 12, 2018; Accepted April 26, 2019; Electronically published December 9, 2019 Online enhancement: appendix. abstract: Sex differences in overall recombination rates are well Nearly all attention to this question has focused on over- known, but little theoretical or empirical attention has been given all map lengths. Achiasmy, in which recombination is lost to how and why sexes differ in their recombination landscapes: the in one sex, has evolved about 30 times, and the loss always patterns of recombination along chromosomes. In the first scientific occurs in the heterogametic sex (Haldane 1922; Huxley review of this phenomenon, we find that recombination is biased to- 1928; Burt et al. 1991). A much more common but less un- ward telomeres in males and more uniformly distributed in females in derstood situation is heterochiasmy, in which both sexes re- most vertebrates and many other eukaryotes. Notable exceptions to combine but at different rates. In these cases, sex differences this pattern exist, however. Fine-scale recombination patterns also fre- quently differ between males and females. The molecular mechanisms in map lengths are not correlated with which sex is hetero- responsible for sex differences remain unclear, but chromatin land- gametic (Burt et al. 1991; Lenormand 2003; Brandvain and scapes play a role. Why these sex differences evolve also is unclear. Hy- Coop 2012), although overall recombination tends to be potheses suggest that they may result from sexually antagonistic selec- higher in females across animals and outcrossing plants (re- tion acting on coding genes and their regulatory elements, meiotic viewed in Lenormand and Dutheil 2005; Brandvain and drive in females, selection during the haploid phase of the life cycle, Coop 2012). -
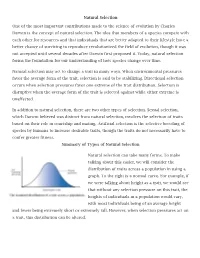
Natural Selection One of the Most Important Contributions Made to The
Natural Selection One of the most important contributions made to the science of evolution by Charles Darwin is the concept of natural selection. The idea that members of a species compete with each other for resources and that individuals that are better adapted to their lifestyle have a better chance of surviving to reproduce revolutionized the field of evolution, though it was not accepted until several decades after Darwin first proposed it. Today, natural selection forms the foundation for our understanding of how species change over time. Natural selection may act to change a trait in many ways. When environmental pressures favor the average form of the trait, selection is said to be stabilizing. Directional selection occurs when selection pressures favor one extreme of the trait distribution. Selection is disruptive when the average form of the trait is selected against while either extreme is unaffected. In addition to natural selection, there are two other types of selection. Sexual selection, which Darwin believed was distinct from natural selection, involves the selection of traits based on their role in courtship and mating. Artificial selection is the selective breeding of species by humans to increase desirable traits, though the traits do not necessarily have to confer greater fitness. Summary of Types of Natural Selection Natural selection can take many forms. To make talking about this easier, we will consider the distribution of traits across a population in using a graph. To the right is a normal curve. For example, if we were talking about height as a trait, we would see that without any selection pressure on this trait, the heights of individuals in a population would vary, with most individuals being of an average height and fewer being extremely short or extremely tall. -

Assortative Mating and Sexual Dimorphism in the Common Tern
Wilson Bull., 98(l), 1986, pp. 93-100 ASSORTATIVE MATING AND SEXUAL DIMORPHISM IN THE COMMON TERN MALCOLM C. COULTER ’ ABSTRACT.-Although male and female Common Terns (Sterna hirundo)are almost iden- tical in plumage and in most external body measurements, males have longer, deeper, and wider bills than do females. Two patterns in the size relationships between mates are dem- onstrated. First, males tend to have larger bills than their mates. This may be explained by random mating, given the sexual dimorphism observed. Second, Common Terns mate assortatively according to bill size. This pattern may result if there is either a year-to-year component or an age component of bill size variation and if first-breeding birds return to the colony after experienced breeders have already established pairbonds and most birds retain their mates from year to year. ReceivedI I Jan. 1985, accepted30 Aug. 1985. Mating patterns may contribute significantly to the genetic structure of bird populations. When similar individuals tend to mate with each other (i.e., mating is positively assortative), progeny are more homozygous, and phenotypic variability in the population may be greater than when mating is either random or disassortative (Falconer 198 1, Halliday 1978, Par- tridge 1983; but see Lande 1977). Positive assortative mating based on the color morphs has been demonstrated for white and blue morphs of the Snow Goose (Chen cuerulescens) (Cooke et al. 1976). This involved discrimination according to a discrete variable (i.e., distinct blue and white morphs). It is more difficult to demonstrate mating patterns according to continuous variables, particularly in wild populations. -

Consequences of the Domestication of Man's Best Friend, The
Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology 289 Consequences of the Domestication of Man’s Best Friend, The Dog SUSANNE BJÖRNERFELDT ACTA UNIVERSITATIS UPSALIENSIS ISSN 1651-6214 UPPSALA ISBN 978-91-554-6854-5 2007 urn:nbn:se:uu:diva-7799 ! "# $ % &''( '!)'' * # * * +# #, "# - #, ./ * 0, &''(, 1 * # * $ 2 3 "# , 4 , &5!, 67 , , 809 !(5:!;:<<7:65<7:<, "# - # * # ;< ''' , " # # - # # = # , # - * # - * * # * * #, "# # * # # # # # 2 # # # # , " # - # - ) # # 94 # > # # , 8 1 # - # ** - # # # 2 * , 3 - * # - # # -# * , "# # * # # # * * * # # # , ? # * , * 1 * * - # *- * , @ - * # * - # -## * * , "# 1 # # # * * # * * , + 2 * # * - # - - # 2 , # > # -# * * - 1 * !" # $ # % # $ # & '( )*# # $+,-./0 # A 0 ./ * &''( 8009 ;6<;:6&;7 809 !(5:!;:<<7:65<7:< ) ))) :((!! B# )CC ,,C D E ) ))) :((!!F All knowledge, the totality of all questions and answers, is contained in the dog. FRANZ KAFKA, Investigations of the dog Cover photos by S Björnerfeldt List of Papers This thesis -

Natural Selection in Action During Speciation
Natural selection in action during speciation Sara Via1 Departments of Biology and Entomology, University of Maryland, College Park, MD 20742 The role of natural selection in speciation, first described by The Spyglass Darwin, has finally been widely accepted. Yet, the nature and time A course of the genetic changes that result in speciation remain mysterious. To date, genetic analyses of speciation have focused almost exclusively on retrospective analyses of reproductive iso- lation between species or subspecies and on hybrid sterility or Intrinsic barriers to Ancestral inviability rather than on ecologically based barriers to gene flow. gene flow evolve… Population Diverged “Good Species” However, if we are to fully understand the origin of species, we Populations must analyze the process from additional vantage points. By studying the genetic causes of partial reproductive isolation be- The Magnifying Glass tween specialized ecological races, early barriers to gene flow can B be identified before they become confounded with other species differences. This population-level approach can reveal patterns that become invisible over time, such as the mosaic nature of the genome early in speciation. Under divergent selection in sympatry, Ancestral Intrinsic barriers to the genomes of incipient species become temporary genetic mo- Population gene flow evolve… Diverged “Good Species” saics in which ecologically important genomic regions resist gene Populations exchange, even as gene flow continues over most of the genome. Analysis of such mosaic genomes suggests that surprisingly large Fig. 1. Two ways to study the process of speciation, which is visualized here genomic regions around divergently selected quantitative trait loci as a continuum of divergence from a variable population to a divergent pair of populations, and on through the evolution of intrinsic barriers to gene flow can be protected from interrace recombination by ‘‘divergence to the recognition of good species. -

Biol2007 - Evolution of Quantitative Characters
1 BIOL2007 - EVOLUTION OF QUANTITATIVE CHARACTERS How do evolutionary biologists measure variation in a typical quantitative character? Let’s use beak size in birds as a typical example. Phenotypic variation (Vp) in a quantitative character Some of the variation in beak size is caused by environmental factors (VE). Some of the variation is caused by genetic factors (VG). If there was no variation in genotype between birds then individuals would differ in beak size only because of different rearing environments. Quantitative geneticists think in terms of how the value of a character in an individual compares to the mean value of the character in the population. The population mean is taken to be a neutral measure and then particular phenotypes are increases or decreases from that value. AT LEVEL OF INDIVIDUAL Phenotype = genotype + environment P = G + E AT LEVEL OF POPULATION Phenotypic variance = genotypic variance + environmental variance VP = VG + VE Genotypic variance is divided into additive, dominance and interaction components VG = VA + VD + V I VA = Additive variance – due to the additive effects of individual alleles (inherited) VD = Dominance variance - reflect the combinations of alleles at each locus (homo- versus (homozygotes versus hetero-zygotes) in any given generation but are not inherited by offspring. An intra-locus effect. VI = Interaction/epistatic variance - reflect epistatic interactions between alleles at different loci. Again not passed onto offspring; they depend on particular combinations of genes in a given generation and are not inherited by offspring. An inter-locus effect. ___________________________________________________________ The following section (italicised) provides the alebgra to backup my assertion that VD and VI are not heritable.