Malformed Vertebrae: a Clinical and Imaging Review
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

M ONITOR a Semi-Annual Data and Research Update Texas Department of Health, Bureau of Epidemiology
Texas Birth Defects M ONITOR A Semi-Annual Data and Research Update Texas Department of Health, Bureau of Epidemiology VOLUME 10, NUMBER 1, June 2004 FROM THE DIRECTOR The web site also has a useful glossary linked to risk factor summaries for a number of birth defects. INTERACTIVE WEB PAGE ALLOWS EASY RESEARCH SYMPOSIUM ACCESS TO TEXAS BIRTH DEFECTS DATA Birth defects data were recently highlighted at the Texas In partnership with Texas Department of Health's Center for Birth Defects Research Symposium on April 9 in San Anto- Health Statistics, birth defects data are now available on the nio. The following speakers provided insight into the causes Texas Health Data web site. Visitors to the site (http://soup- of birth defects: fin.tdh.state.tx.us/) will be able to query data from the Texas Birth Defects Registry. Linking Birth Defects and the Environment, with Prelimi- nary Findings from an Air Pollution Study in Texas (Peter The Registry uses active surveillance to collect information Langlois, Ph.D., TBDMD and Suzanne Gilboa, M.H.S., U.S. about infants and fetuses with birth defects, born to women Environmental Protection Agency) residing in Texas. Data are presented for 49 defect catego- ries, plus a category for “infants and fetuses with any moni- Neural Tube Defects: Multiple Risk Factors Among the tored birth defect” beginning with deliveries in 1999, when Texas-Mexico Border Population (Lucina Suarez, Ph.D., the Texas Birth Defects Registry became statewide. Texas Department of Health) The Embryonic Consequences of Abnormal -

CASE REPORT Congenital Posterior Atlas Defect Associated with Anterior
Acta Orthop. Belg., 2007, 73, 282-285 CASE REPORT Congenital posterior atlas defect associated with anterior rachischisis and early cervical degenerative disc disease : A case study and review of the literature Dritan PASKU, Pavlos KATONIS, Apostolos KARANTANAS, Alexander HADJIPAVLOU From the University of Crete Heraklion, Greece A rare case of a wide congenital atlas defect is report- diagnosed posterior atlas defect coexisting with an ed. A 25 year-old woman was admitted after com- anterior rachischisis, presenting with radicular arm plaints of radicular pain in the right arm. pain resistant to conservative therapy. In addition, a Radiographs incidentally revealed aplasia of the pos- review of the literature is presented with emphasis terior arch of the atlas together with anterior rachis- on the possibility of the association between the chisis. A review of the literature is presented and a atlas defect and early disc degeneration. possible association with early disc degeneration is discussed. CASE REPORT Keywords : spine ; congenital disorders ; computed tomography ; MR imaging ; disc degeneration. A 25 year-old woman presented with neck pain radiating to the right arm over the last 5 days. She also reported intermittent neck and arm pain for the INTRODUCTION past 4 years. The patient had consulted in our hos- pital for an episode of cervical pain one year previ- Malformations of the atlas are relatively rare and ously without arm pain but was discharged from exhibit a wide range including aplasia, hypoplasia the emergency department without any radiological and various arch clefts (2, 15). The reported inci- examination. Her symptoms deteriorated with neck dence in a large study of 1,613 autopsies with flexion, with pain referred to the upper thoracic regard to presence of congenital aplasia is 4% for the posterior arch and 0.1% for the anterior arch (5- 8). -

Anencephalic Fetus with Craniospinal Rachischisis – Case Report
Case Study International Journal of Research - GRANTHAALAYAH ISSN (Online): 2350-0530 May 2021 9(5), 24–29 ISSN (Print): 2394-3629 ANENCEPHALIC FETUS WITH CRANIOSPINAL RACHISCHISIS – CASE REPORT 1 Ayse KONAC 1Gelisim University Health Sciences, Istanbul, Turkey ABSTRACT Anencephaly, in which a substantial part of the brain, skull, or scalp is miss- ing, is a lethal neural tube defect (NTD) that occurs during the fourth week of pregnancy after failed cranial neuropore closure. One in every 1,000 births is anencephalic, and newborns with this NTD are not viable or treatable. Associ- ated with anencephaly is rachischisis, or severe incomplete neural tube closure and exposure of the spinal cord. Ultrasonography can quickly diagnose anen- cephaly. Like other NTDs, nutritional and environmental factors both play a role in the development of anencephaly. Here, we report and discuss an unusual case of a 12-week gestation anencephalic fetus with craniospinal rachischisis and its embryological roots. In our case, except from the low socio-economic life of the patient, the Received 18 April 2021 absence of a predisposing factor that could cause such an anomaly, the abor- Accepted 4 May 2021 tion being in the irst trimester and the occurrence in the irst pregnancy of the Published 31 May 2021 patient as a result of 5-year infertility made us think that pathology examination Corresponding Author of the abortus material is important in complet or incomplete abortions. Ayse KONAC, ayse.konac1@gmail. com DOI 10.29121/ Keywords: Anencephaly, Neural Tube Defect, Rachischisis, İncomplet Abortion, granthaalayah.v9.i5.2021.3899 First Trimester Funding: This research received no speciic grant from any funding agency in the public, commercial, or not-for-proit sectors. -

WHO Manual of Diagnostic Imaging Radiographic Anatomy and Interpretation of the Musculoskeletal System
The WHO manual of diagnostic imaging Radiographic Anatomy and Interpretation of the Musculoskeletal System Editors Harald Ostensen M.D. Holger Pettersson M.D. Authors A. Mark Davies M.D. Holger Pettersson M.D. In collaboration with F. Arredondo M.D., M.R. El Meligi M.D., R. Guenther M.D., G.K. Ikundu M.D., L. Leong M.D., P. Palmer M.D., P. Scally M.D. Published by the World Health Organization in collaboration with the International Society of Radiology WHO Library Cataloguing-in-Publication Data Davies, A. Mark Radiography of the musculoskeletal system / authors : A. Mark Davies, Holger Pettersson; in collaboration with F. Arredondo . [et al.] WHO manuals of diagnostic imaging / editors : Harald Ostensen, Holger Pettersson; vol. 2 Published by the World Health Organization in collaboration with the International Society of Radiology 1.Musculoskeletal system – radiography 2.Musculoskeletal diseases – radiography 3.Musculoskeletal abnormalities – radiography 4.Manuals I.Pettersson, Holger II.Arredondo, F. III.Series editor: Ostensen, Harald ISBN 92 4 154555 0 (NLM Classification: WE 141) The World Health Organization welcomes requests for permission to reproduce or translate its publications, in part or in full. Applications and enquiries should be addressed to the Office of Publications, World Health Organization, CH-1211 Geneva 27, Switzerland, which will be glad to provide the latest information on any changes made to the text, plans for new editions, and reprints and translations already available. © World Health Organization 2002 Publications of the World Health Organization enjoy copyright protection in accordance with the provisions of Protocol 2 of the Universal Copyright Convention. All rights reserved. -

Musculo-Skeletal System
Musculo-Skeletal System (Trunk, Limbs, and Head) somite: ectoderm dermatome General Statements: myotome Bilaterally, paraxial mesoderm become sclerotome neural crest somites and somitomeres. (Somitomeres develop ros- intermediate tral to the notochord in the head. They are like somites, but mesoderm neural tube smaller and less distinctly organized.) The mesoderm somatic mesoderm comprising each somite differentiates into three notochord regions: endoderm aorta — dermatome (lateral) which migrates to form dermis of the skin coelom — sclerotome (medial) forms most of the splanchnic mesoderm axial skeleton (vertebrae, ribs, and base of the skull). Mesoderm Regions — myotome (middle) forms skeletal mus- culature. Individual adult muscles are produced by merger of adjacent myotomes. Note: Nerves make early connections with adjacent myotomes and dermatomes, establishing a segmental innervation pattern. As myotome/dermatome cells migrate to assume adult positions, the segmental nerve supply must follow along to maintain its connection to the innervation target. (Recurrent laryngeal & phrenic nerves travel long distances because their targets migrated far away.) Skin. Consists of dermis and epidermis. Epidermis, including hair follicles & glands, is derived from ectoderm. Neural crest cells migrate into epidermis and become melanocytes. (Other neural crest cells become tactile disc receptors.) Dermis arises from mesoderm (dermatomes of somites). Each dermatome forms a continu- ous area of skin innervated by one spinal nerve. Because adjacent dermatomes overlap, a locus of adult skin is formed by 2 or 3 dermatomes, and innervated by 2 or 3 spinal nerves. Muscle. Muscles develop from mesoderm, except for muscles of the iris which arise from optic cup ectoderm. Cardiac and smooth muscles originate from splanchnic mesoderm. -
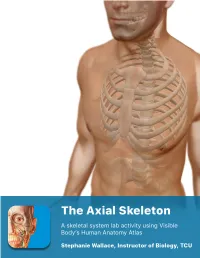
Lab Manual Axial Skeleton Atla
1 PRE-LAB EXERCISES When studying the skeletal system, the bones are often sorted into two broad categories: the axial skeleton and the appendicular skeleton. This lab focuses on the axial skeleton, which consists of the bones that form the axis of the body. The axial skeleton includes bones in the skull, vertebrae, and thoracic cage, as well as the auditory ossicles and hyoid bone. In addition to learning about all the bones of the axial skeleton, it is also important to identify some significant bone markings. Bone markings can have many shapes, including holes, round or sharp projections, and shallow or deep valleys, among others. These markings on the bones serve many purposes, including forming attachments to other bones or muscles and allowing passage of a blood vessel or nerve. It is helpful to understand the meanings of some of the more common bone marking terms. Before we get started, look up the definitions of these common bone marking terms: Canal: Condyle: Facet: Fissure: Foramen: (see Module 10.18 Foramina of Skull) Fossa: Margin: Process: Throughout this exercise, you will notice bold terms. This is meant to focus your attention on these important words. Make sure you pay attention to any bold words and know how to explain their definitions and/or where they are located. Use the following modules to guide your exploration of the axial skeleton. As you explore these bones in Visible Body’s app, also locate the bones and bone markings on any available charts, models, or specimens. You may also find it helpful to palpate bones on yourself or make drawings of the bones with the bone markings labeled. -

Closed Spinal Dysraphism and Tethered Cord
ACNRSO14_Layout 1 04/09/2014 22:14 Page 28 NEUROSURGERY ARTICLE Ruth-Mary deSouza trained in medicine at Closed Spinal Dysraphism Guy’s, Kings and St Thomas Medical School and graduated in 2008. She entered the London and Tethered Cord Neurosurgery training programme in 2010 and is currently an ST5 trainee on the South Thames Syndrome: A Review of Neurosurgery programme. David Frim Multidisciplinary Team Management is Professor of Surgery, Neurology and Paediatrics at the University of Chicago. He is an Summary internationally recognised • Embryology of spinal dysraphism clinical Neurosurgeon and Neurosciences Researcher • Clinical features of tethered cord syndrome who specialises in the care • Multidisciplinary management of closed spinal dysraphism of children and adults with congenital neurosurgical problems. Currently, Dr Frim serves as principal investigator on laboratory studies related to neural injury and clinical studies focusing on Abstract outcomes after treatment of congenital anomalies of the nervous system especially as related to cognition. The initial diagnosis as well as the long term management of occult spinal dysraphism and Dr Frim is joint senior author of the article. tethered spinal cord is often managed by a large number of healthcare professionals including Paediatricians, GPs, Neurologists, Neurosurgeons, Rehabilitation Physicians and Paige Terrien Church Therapists. We review the entity of spinal dysraphism. An approach to the evaluation and is an Assistant Professor of diagnosis of these entities is subsequently discussed. In addition, concepts involved in the Paediatrics at the pathophysiology, neurosurgical repair, and outcome are presented in the context of postop - University of Toronto. She is the Director of the erative management issues that rely upon the knowledge of all professionals who may Neonatal Follow Up Clinic encounter these patients. -

The Axial Skeleton – Hyoid Bone
Marieb’s Human Anatomy and Physiology Ninth Edition Marieb Hoehn Chapter 7 The Axial and Appendicular Skeleton Lecture 14 1 Lecture Overview • Axial Skeleton – Hyoid bone – Bones of the orbit – Paranasal sinuses – Infantile skull – Vertebral column • Curves • Intervertebral disks –Ribs 2 The Axial Skeleton – Hyoid Bone Figure from: Saladin, Anatomy & Physiology, McGraw Hill, 2007 Suspended from the styloid processes of the temporal bones by ligaments and muscles The hyoid bone supports the larynx and is the site of attachment for the muscles of the larynx, pharynx, and tongue 3 1 Axial Skeleton – the Orbit See Fig. 7.6.1 in Martini and Fig. 7.20 in Figure: Martini, Right Hole’s Textbook Anatomy & Physiology, Optic canal – Optic nerve; Prentice Hall, 2001 opthalmic artery Superior orbital fissure – Oculomotor nerve, trochlear nerve, opthalmic branch of trigeminal nerve, abducens nerve; opthalmic vein F Inferior orbital fissure – Maxillary branch of trigeminal nerve E Z S L Infraorbital groove – M N Infraorbital nerve, maxillary branch of trigeminal nerve, M infraorbital artery Lacrimal sulcus – Lacrimal sac and tearduct *Be able to label a diagram of the orbit for lecture exam 4 Nasal Cavities and Sinuses Paranasal sinuses are air-filled, Figure: Martini, mucous membrane-lined Anatomy & Physiology, chambers connected to the nasal Prentice Hall, 2001 cavity. Superior wall of nasal cavities is formed by frontal, ethmoid, and sphenoid bones Lateral wall of nasal cavities formed by maxillary and lacrimal bones and the conchae Functions of conchae are to create swirls, turbulence, and eddies that: - direct particles against mucus - slow air movement so it can be warmed and humidified - direct air to superior nasal cavity to olfactory receptors 5 Axial Skeleton - Sinuses Sinuses are lined with mucus membranes. -

BODY MOVEMENTS and LOCOMOTION in HUMAN BEINGS Locomotion Is the Main Characteristic Feature That Distinguishes Animals from Plants
CHAPTER 8 MODULE 2 RECAP Movement is when a living organism moves a body part or parts without changing the position of the organism Animals carry out many activities which involve the displacement of an organism from its original position. This activity carried out by the organism is called locomotion. MOVABLE JOINT- Joints where bones can move IMMOVABLE JOINT-Joints where bones cannot move. Types of movable joints: Pivot joint, Ball and Socket joint , Hinge joint, Gliding joints BODY MOVEMENTS AND LOCOMOTION IN HUMAN BEINGS Locomotion is the main characteristic feature that distinguishes animals from plants. In human beings, various body movements and locomotion are controlled by skeletal system and muscular system.. The skeletal system is made of bones and muscular system is made of muscles. SKELETAL SYSTEM SKELETAL SYSTEM CONTINUED…. The system that supports the overall body by providing a definite shape and helps in the movement is known as skeletal system. Skeleton is the framework of bones in the body. The adult human skeleton consists of 206 bones. They are very hard on the outer side and soft on the inner side. FUNCTIONS OF SKELETON FUNCTIONS OF SKELETON Protection to vital organs. Support to body. Shape to body. Movement of body organs HUMAN SKELETAL SYSTEM CONTINUED…. AXIAL SKELETON The axial skeleton is made of following parts. Skull Vertebral column(backbone) Sternum(breast bone) AXIAL SKELETON APPENDICULAR SKELETON The appendicular skeleton consists of upper and lower limbs and girdles. The bones that provide support and space for the movement of limb bones are known as girdles. Pectoral girdle is located on upper part of body. -

Axial Skeleton •The Basic Features of the Human Skeleton Have Been Shaped by Evolution, but the Detailed Characteristics of Each Bone Reflect the Stresses Put on It
The Axial Skeleton •The basic features of the human skeleton have been shaped by evolution, but the detailed characteristics of each bone reflect the stresses put on it . As a result, the skeleton changes during its lifetime. The skeletal system is divided into: 1. Axial Division: bones of the body’s axis (skulll, ribs, vertebrae) 2. Appendicular Division: bones appended to the axial bones of the body (arms, legs, shoulders, hands, feet, etc.) There are roughly 80 bones in the Axial skeleton, and they form the bones of the longitudinal axis of the body (roughly 40% of the bones in the human body) Skull Bones: 8 cranial, 18 facial Associated Skull Bones: 6 auditory ossicles, 1 hyoid bone Thoracic Bones: 1 sternum, 24 ribs Vertebral Bones: 24 vertebrae, 1 sacrum, 1 coccyx 1 The functions of the axial skeleton are: 1. Create a framework to support and protect organs in the dorsal and ventral cavities 2. Provide extensive surface area for the attachment of muscles that: a. adjjpust the position of the head, neck and trunk b. perform respiratory movement c. stabilize or position the appendicular skeleton • The joints of the axial skeleton are limiting in terms of movement, but are VERY strong and heavily reinforced with ligaments. The Skull •The bones of the skull protect the brain and guard the entrances to the digestive and respiratory systems. The skull contains 22 bones. Eight bones form the majority of the cranium or braincase: a. occipital b. parietal (L-R) c. frontal d. temporal (L- R) e. sphenoid (L-R) •These bones along with the ethmoid (a facial bone) completely enclose the brain case. -

Anencephalic Fetus with Craniospinal Rachischisis - Case Report
IP Indian Journal of Anatomy and Surgery of Head, Neck and Brain 2019;5(4):124–126 Content available at: iponlinejournal.com IP Indian Journal of Anatomy and Surgery of Head, Neck and Brain Journal homepage: www.innovativepublication.com Case Report Anencephalic fetus with craniospinal rachischisis - Case report Sangeeta S Kotrannavar1, Vijaykumar S Kotrannavar2,* 1Dept. of Anatomy, USM- KLE International Medical Programme, Belgaum, India 2Shri JGCHS Ayurvedic Medical College, Ghataprabha, Karnataka, India ARTICLEINFO ABSTRACT Article history: Anencephaly is a severe neural tube defect (NTD) caused by failure of closure in the cranial neuropore Received 04-12-2019 during fourth week of pregnancy. As a result, major portion of the brain, skull and scalp is absent. Accepted 22-12-2019 Anencephaly may be associated with rachischisis, where defective neural tube closure is extensive and Available online 24-01-2020 spinal cord is exposed. Overall incidence of anencephaly is one in every 1000 births. It can be easily diagnosed by ultrasonography. Anencephaly newborns are not viable nor treatable and classified as lethal NTDs. Nutritional and environmental factors play a role in production of NTDs. Here we report and Keywords: discuss a rare case of anencephalic fetus with craniospinal rachischisis of 25 weeks of gestation and their Anencephaly embryological origin. Neural tube defect Rachischisis © 2019 Published by Innovative Publication. This is an open access article under the CC BY-NC-ND license (https://creativecommons.org/licenses/by/4.0/) 1. Introduction forms brain and caudal part develops into spinal cord. NTDs result from abnormal closure of neural folds in third and Anencephaly is a congenital severe lethal neural tube fourth week of development. -

Axial Skeleton List for API
Axial Skeleton List for API Sutures Coronal Lambdoidal Squamous Sagittal Bones of the Skull Frontal Bone Parietal Bone Temporal Bone Occipital Bone Zygomatic Bone Zygomatic Arch Zygomatic Process Mandible (mental foramen) Maxilla Nasal Bone Vomer Bone Ethmoid Bone (Crista galli) Foramen magnum Sella turcica External auditory (acoustic) meatus Mastoid Process Styloid Process Superior nuchal line Inferior nuchal line External occipital protuberance (EOP) Sternum (xyphoid process) Ribs Sacrum Coccyx Dr. Tarsha Smith Page 1 Axial Skeleton List for API (continued) Vertebrae Atlas (superior articular facet; posterior tubercle) Axis (dens or odontoid process) Cervical, Thoracic, Lumbar Know following terms on all vertebrae, if present: Facet, Pedicle, Spinous, Body, Transverse process, Lamina Appendicular Skeleton List for API Clavicle Conoid tubercle Scapula Acromion Medial border Coracoid process Lateral border Glenoid cavity Subscapular fossa Spine Infraspinous fossa Humerus Head Capitulum Surgical neck Trochlea Greater tubercle Olecranon fossa Lesser tubercle Coronoid fossa Epicondyles (lateral & medial) Radius Tuberosity of radius Articular facets Styloid process Head & neck Ulna Trochlear notch Radial notch of ulna Coronoid process Hand Phalanges Metacarpals Carpals: Scaphoid, Lunate, Triquetrium, Pisiform, Trapezium, Trapezoid, Capitate, Hamate Dr. Tarsha Smith Page 2 Appendicular Skeleton List for API (continued) Pelvis Ilium Crest Anterior superior Anterior inferior Ischium Body Ramus Spine Pubis Pubic symphysis Obturator foramen Acetabulum Femur Fovea capitis Greater trochanter Head Neck Lesser trochanter Linea aspera Epicondyles (medial & lateral) Condyles (medial & lateral) Intercondylar fossa Patella (kneecap) Tibia Condyle (medial & lateral) Tibial tuberosity Medial malleolus Fibula Lateral malleolus Apex Foot Metatarsals Phalanx Tarsals: Cuneiforms, Navicular, talus, cuboid, calcaneus Dr. Tarsha Smith Page 3 .