Musculo-Skeletal System
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mesenchymal Stem Cells in Combination with Hyaluronic Acid
www.nature.com/scientificreports OPEN Mesenchymal Stem Cells in Combination with Hyaluronic Acid for Articular Cartilage Defects Received: 1 August 2017 Lang Li1, Xin Duan1, Zhaoxin Fan2, Long Chen1,3, Fei Xing1, Zhao Xu4, Qiang Chen2,5 & Accepted: 19 April 2018 Zhou Xiang1 Published: xx xx xxxx Mesenchymal stem cells (MSCs) and hyaluronic acid (HA) have been found in previous studies to have great potential for medical use. This study aimed to investigate the therapeutic efects of bone marrow mesenchymal stem cells (BMSCs) combined with HA on articular cartilage repair in canines. Twenty-four healthy canines (48 knee-joints), male or female with weight ranging from 5 to 6 kg, were operated on to induce cartilage defect model and divided into 3 groups randomly which received diferent treatments: BMSCs plus HA (BMSCs-HA), HA alone, and saline. Twenty-eight weeks after treatment, all canines were sacrifced and analyzed by gross appearance, magnetic resonance imaging (MRI), hematoxylin-eosin (HE) staining, Masson staining, toluidine blue staining, type II collagen immunohistochemistry, gross grading scale and histological scores. MSCs plus HA regenerated more cartilage-like tissue than did HA alone or saline. According to the macroscopic evaluation and histological assessment score, treatment with MSCs plus HA also lead to signifcant improvement in cartilage defects compared to those in the other 2 treatment groups (P < 0.05). These fndings suggested that allogeneic BMSCs plus HA rather than HA alone was efective in promoting the formation of cartilage-like tissue for repairing cartilage defect in canines. Articular cartilage is composed of chondrocyte and extracellular matrix and has an important role in joint move- ment including lubrication, shock absorption and conduction. -

(AMIC) Compared to Microfractures for Chondral Defects of the Talar Shoulder: a Five-Year Follow-Up Prospective Cohort Study
life Communication Autologous Matrix Induced Chondrogenesis (AMIC) Compared to Microfractures for Chondral Defects of the Talar Shoulder: A Five-Year Follow-Up Prospective Cohort Study Filippo Migliorini 1 , Jörg Eschweiler 1, Nicola Maffulli 2,3,4,5,* , Hanno Schenker 1, Arne Driessen 1 , Björn Rath 1,6 and Markus Tingart 1 1 Department of Orthopedics and Trauma Surgery, University Clinic Aachen, RWTH Aachen University Clinic, 52064 Aachen, Germany; [email protected] (F.M.); [email protected] (J.E.); [email protected] (H.S.); [email protected] (A.D.); [email protected] (B.R.); [email protected] (M.T.) 2 School of Pharmacy and Bioengineering, Keele University School of Medicine, Staffordshire ST4 7QB, UK 3 Barts and the London School of Medicine and Dentistry, London E1 2AD, UK 4 Centre for Sports and Exercise Medicine, Queen Mary University of London, Mile End Hospital, London E1 4DG, UK 5 Department of Orthopedics, Klinikum Wels-Grieskirchen, A-4600 Wels, Austria 6 Department of Medicine, Surgery and Dentistry, University of Salerno, 84081 Baronissi, Italy * Correspondence: [email protected] Abstract: Introduction: Many procedures are available to manage cartilage defects of the talus, Citation: Migliorini, F.; Eschweiler, J.; including microfracturing (MFx) and Autologous Matrix Induced Chondrogenesis (AMIC). Whether Maffulli, N.; Schenker, H.; Driessen, AMIC or MFx are equivalent for borderline sized defects of the talar shoulder is unclear. Thus, the A.; Rath, B.; Tingart, M. Autologous present study compared the efficacy of primary isolated AMIC versus MFx for borderline sized Matrix Induced Chondrogenesis focal unipolar chondral defects of the talar shoulder at midterm follow-up. -

Applications of Chondrocyte-Based Cartilage Engineering: an Overview
Hindawi Publishing Corporation BioMed Research International Volume 2016, Article ID 1879837, 17 pages http://dx.doi.org/10.1155/2016/1879837 Review Article Applications of Chondrocyte-Based Cartilage Engineering: An Overview Abdul-Rehman Phull,1 Seong-Hui Eo,1 Qamar Abbas,1 Madiha Ahmed,2 and Song Ja Kim1 1 Department of Biological Sciences, College of Natural Sciences, Kongju National University, Gongjudaehakro 56, Gongju 32588, Republic of Korea 2Department of Pharmacy, Quaid-i-Azam University, Islamabad 45320, Pakistan Correspondence should be addressed to Song Ja Kim; [email protected] Received 14 May 2016; Revised 24 June 2016; Accepted 26 June 2016 Academic Editor: Magali Cucchiarini Copyright © 2016 Abdul-Rehman Phull et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Chondrocytes are the exclusive cells residing in cartilage and maintain the functionality of cartilage tissue. Series of biocomponents such as different growth factors, cytokines, and transcriptional factors regulate the mesenchymal stem cells (MSCs) differentiation to chondrocytes. The number of chondrocytes and dedifferentiation are the key limitations in subsequent clinical application of the chondrocytes. Different culture methods are being developed to overcome such issues. Using tissue engineering and cell based approaches, chondrocytes offer prominent therapeutic option specifically in orthopedics for cartilage repair and to treat ailments such as tracheal defects, facial reconstruction, and urinary incontinence. Matrix-assisted autologous chondrocyte transplantation/implantation is an improved version of traditional autologous chondrocyte transplantation (ACT) method. An increasing number of studies show the clinical significance of this technique for the chondral lesions treatment. -

Embryonic Cell That Forms Cartilage Medical Term
Embryonic Cell That Forms Cartilage Medical Term Unexploited Gordie languishes: he scumbles his initiatives atweel and esthetically. When Nate gestate his niggardliness Grecizing not post-free enough, is Mikhail windowless? Ship-rigged or millionth, Edgar never enshrining any millionairesses! The crest cell phenotype research in record area forms the body of shift review the Table 1. Where and repair differs substantially augments the embryonic cartilage tissue types of its tension adaptation and cells? In both types for medicine to that cartilage. Cells turn into differentiated stem cells that trace specific tissues and organs. Ambiguous cells the emergence of daughter stem a concept in. Mesenchymal Chondrosarcoma NORD National. Blood cells Chondro Oma Cartilage Tumor Arthro Joints Cartilage creates a. Can disturb blood cells and stromal which manufacture produce fat cartilage and bone. Label by following from NURSING 3345 at University of Texas Medical Branch. Body mostly a laboratory stem cells divide that form more cells called daughter cells. Guidelines for Human Embryonic Stem Cell with Brown. Abstract The skeletal system is formed of bones and cartilage which are. Each tissue cartilage bone and skeletal muscle goes through my different. Medical terms UCL. Please note love the definitions are moving given an explain another word found also a. Definition Stem cells are cells which feature not yet developed a special. The term totipotent refer down the grief that they ever total potential to. Stem from Research Uses Types & Examples Healthline. For cardiac muscle cells and was still pluripotent stem cells may also structures and cartilage that embryonic cell forms a primitive connective tissue physiology as well as macrophages are adequately informed consent. -

Autologous Matrix-Induced Chondrogenesis and Generational Development of Autologous Chondrocyte Implantation
Autologous Matrix-Induced Chondrogenesis and Generational Development of Autologous Chondrocyte Implantation Hajo Thermann, MD, PhD,* Christoph Becher, MD,† Francesca Vannini, MD, PhD,‡ and Sandro Giannini, MD‡ The treatment of osteochondral defects of the talus is still controversial. Matrix-guided treatment options for covering of the defect with a scaffold have gained increasing popularity. Cellular-based autologous chondrocyte implantation (ACI) has undergone a generational development overcoming the surgical drawbacks related to the use of the periosteal flap over time. As ACI is associated with high costs and limited in availability, autologous matrix-induced chondrogenesis, a single-step procedure combining microfracturing of the subchondral bone to release bone marrow mesenchymal stem cells in combination with the coverage of an acellular matrix, has gained increasing popularity. The purposes of this report are to present the arthroscopic approach of the matrix-guided autologous matrix-induced chondrogenesis technique and generational development of ACI in the treatment of chondral and osteochon- dral defects of the talus. Oper Tech Orthop 24:210-215 C 2014 Elsevier Inc. All rights reserved. KEYWORDS cartilage, defect, ankle, talus, AMIC, ACI Introduction Cartilage repair may be obtained by cartilage replacement: (OATS, mosaicplasty) or with techniques aimed to generate a hondral and osteochondral lesions are defects of the newly formed cartilage such as microfracture or autologous Ccartilaginous surface and underlying subchondral bone of chondrocyte implantation (ACI).9-17 the talar dome. These defects are often caused by a single or Arthroscopic debridement and bone marrow stimulation multiple traumatic events, mostly inversion or eversion ankle using the microfracture technique has proven to be an 1,2 sprains in young, active patients. -

Study Guide Medical Terminology by Thea Liza Batan About the Author
Study Guide Medical Terminology By Thea Liza Batan About the Author Thea Liza Batan earned a Master of Science in Nursing Administration in 2007 from Xavier University in Cincinnati, Ohio. She has worked as a staff nurse, nurse instructor, and level department head. She currently works as a simulation coordinator and a free- lance writer specializing in nursing and healthcare. All terms mentioned in this text that are known to be trademarks or service marks have been appropriately capitalized. Use of a term in this text shouldn’t be regarded as affecting the validity of any trademark or service mark. Copyright © 2017 by Penn Foster, Inc. All rights reserved. No part of the material protected by this copyright may be reproduced or utilized in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system, without permission in writing from the copyright owner. Requests for permission to make copies of any part of the work should be mailed to Copyright Permissions, Penn Foster, 925 Oak Street, Scranton, Pennsylvania 18515. Printed in the United States of America CONTENTS INSTRUCTIONS 1 READING ASSIGNMENTS 3 LESSON 1: THE FUNDAMENTALS OF MEDICAL TERMINOLOGY 5 LESSON 2: DIAGNOSIS, INTERVENTION, AND HUMAN BODY TERMS 28 LESSON 3: MUSCULOSKELETAL, CIRCULATORY, AND RESPIRATORY SYSTEM TERMS 44 LESSON 4: DIGESTIVE, URINARY, AND REPRODUCTIVE SYSTEM TERMS 69 LESSON 5: INTEGUMENTARY, NERVOUS, AND ENDOCRINE S YSTEM TERMS 96 SELF-CHECK ANSWERS 134 © PENN FOSTER, INC. 2017 MEDICAL TERMINOLOGY PAGE III Contents INSTRUCTIONS INTRODUCTION Welcome to your course on medical terminology. You’re taking this course because you’re most likely interested in pursuing a health and science career, which entails proficiencyincommunicatingwithhealthcareprofessionalssuchasphysicians,nurses, or dentists. -

Comparison of Juvenile Allogenous Articular Cartilage and Bone
FAIXXX10.1177/1071100717746627Foot & Ankle InternationalKarnovsky et al 746627research-article2018 Article Foot & Ankle International® 2018, Vol. 39(4) 393 –405 Comparison of Juvenile Allogenous © The Author(s) 2018 Reprints and permissions: sagepub.com/journalsPermissions.nav Articular Cartilage and Bone Marrow DOI:https://doi.org/10.1177/1071100717746627 10.1177/1071100717746627 Aspirate Concentrate Versus Microfracture journals.sagepub.com/home/fai With and Without Bone Marrow Aspirate Concentrate in Arthroscopic Treatment of Talar Osteochondral Lesions In-Depth Sydney C. Karnovsky, BA1, Bridget DeSandis, BA1, Amgad M. Haleem, MD, PhD2,3, Carolyn M. Sofka, MD4, Martin O’Malley, MD5, and Mark C. Drakos, MD5 Abstract Background: The purpose of this study was to compare the functional and radiographic outcomes of patients who received juvenile allogenic chondrocyte implantation with autologous bone marrow aspirate (JACI-BMAC) for treatment of talar osteochondral lesions with those of patients who underwent microfracture (MF). Methods: A total of 30 patients who underwent MF and 20 who received DeNovo NT for JACI-BMAC treatment between 2006 and 2014 were included. Additionally, 17 MF patients received supplemental BMAC treatment. Retrospective chart review was performed and functional outcomes were assessed pre- and postoperatively using the Foot and Ankle Outcome Score and Visual Analog pain scale. Postoperative magnetic resonance images were reviewed and evaluated using a modified Magnetic Resonance Observation of Cartilage Tissue (MOCART) score. Average follow-up for functional outcomes was 30.9 months (range, 12-79 months). Radiographically, average follow-up was 28.1 months (range, 12-97 months). Results: Both the MF and JACI-BMAC showed significant pre- to postoperative improvements in all Foot and Ankle Outcome Score subscales. -

SOX9 Keeps Growth Plates and Articular Cartilage Healthy by Inhibiting Chondrocyte Dedifferentiation/ Osteoblastic Redifferentiation
SOX9 keeps growth plates and articular cartilage healthy by inhibiting chondrocyte dedifferentiation/ osteoblastic redifferentiation Abdul Haseeba,1, Ranjan Kca,1, Marco Angelozzia, Charles de Charleroya, Danielle Ruxa, Robert J. Towerb, Lutian Yaob, Renata Pellegrino da Silvac, Maurizio Pacificia, Ling Qinb, and Véronique Lefebvrea,2 aDivision of Orthopaedic Surgery, Children’s Hospital of Philadelphia, Philadelphia, PA 19104; bDepartment of Orthopaedic Surgery, University of Pennsylvania, Philadelphia, PA 19104; and cCenter for Applied Genomics, Children’s Hospital of Philadelphia, Philadelphia, PA 19104 Edited by Denis Duboule, University of Geneva, Geneva, Switzerland, and approved January 13, 2021 (received for review September 19, 2020) Cartilage is essential throughout vertebrate life. It starts develop- The skeleton is a model system to study cell fate and differ- ing in embryos when osteochondroprogenitor cells commit to entiation mechanisms. It arises developmentally from multi- chondrogenesis, activate a pancartilaginous program to form carti- potent mesenchymal cells, often called osteochondroprogenitors. laginous skeletal primordia, and also embrace a growth-plate pro- Guided by spatiotemporal cues, these cells commit to chondro- gram to drive skeletal growth or an articular program to build genesis or osteoblastogenesis to build cartilage or bone, respec- permanent joint cartilage. Various forms of cartilage malformation tively (5–7). Cartilage exists in several forms. Articular cartilage and degeneration diseases afflict humans, but underlying mecha- (AC) is a mostly resting tissue that protects opposing bone ends nisms are still incompletely understood and treatment options sub- in synovial joints throughout life, whereas growth plates (GPs) optimal. The transcription factor SOX9 is required for embryonic are transient, dynamic structures that drive skeletal growth while chondrogenesis, but its postnatal roles remain unclear, despite evi- being gradually replaced by bone (endochondral ossification). -

The Epiphyseal Plate: Physiology, Anatomy, and Trauma*
3 CE CREDITS CE Article The Epiphyseal Plate: Physiology, Anatomy, and Trauma* ❯❯ Dirsko J. F. von Pfeil, Abstract: This article reviews the development of long bones, the microanatomy and physiology Dr.med.vet, DVM, DACVS, of the growth plate, the closure times and contribution of different growth plates to overall growth, DECVS and the effect of, and prognosis for, traumatic injuries to the growth plate. Details on surgical Veterinary Specialists of Alaska Anchorage, Alaska treatment of growth plate fractures are beyond the scope of this article. ❯❯ Charles E. DeCamp, DVM, MS, DACVS athologic conditions affecting epi foramen. Growth factors and multipotent Michigan State University physeal (growth) plates in imma stem cells support the formation of neo ture animals may result in severe natal bone consisting of a central marrow P 2 orthopedic problems such as limb short cavity surrounded by a thin periosteum. ening, angular limb deformity, or joint The epiphysis is a secondary ossifica incongruity. Understanding growth plate tion center in the hyaline cartilage forming anatomy and physiology enables practic the joint surfaces at the proximal and distal At a Glance ing veterinarians to provide a prognosis ends of the bones. Secondary ossification Bone Formation and assess indications for surgery. Injured centers can appear in the fetus as early Page E1 animals should be closely observed dur as 28 days after conception1 (TABLE 1). Anatomy of the Growth ing the period of rapid growth. Growth of the epiphysis arises from two Plate areas: (1) the vascular reserve zone car Page E2 Bone Formation tilage, which is responsible for growth of Physiology of the Growth Bone is formed by transformation of con the epiphysis toward the joint, and (2) the Plate nective tissue (intramembranous ossifica epiphyseal plate, which is responsible for Page E4 tion) and replacement of a cartilaginous growth in bone length.3 The epiphyseal 1 Growth Plate Closure model (endochondral ossification). -

WHO Manual of Diagnostic Imaging Radiographic Anatomy and Interpretation of the Musculoskeletal System
The WHO manual of diagnostic imaging Radiographic Anatomy and Interpretation of the Musculoskeletal System Editors Harald Ostensen M.D. Holger Pettersson M.D. Authors A. Mark Davies M.D. Holger Pettersson M.D. In collaboration with F. Arredondo M.D., M.R. El Meligi M.D., R. Guenther M.D., G.K. Ikundu M.D., L. Leong M.D., P. Palmer M.D., P. Scally M.D. Published by the World Health Organization in collaboration with the International Society of Radiology WHO Library Cataloguing-in-Publication Data Davies, A. Mark Radiography of the musculoskeletal system / authors : A. Mark Davies, Holger Pettersson; in collaboration with F. Arredondo . [et al.] WHO manuals of diagnostic imaging / editors : Harald Ostensen, Holger Pettersson; vol. 2 Published by the World Health Organization in collaboration with the International Society of Radiology 1.Musculoskeletal system – radiography 2.Musculoskeletal diseases – radiography 3.Musculoskeletal abnormalities – radiography 4.Manuals I.Pettersson, Holger II.Arredondo, F. III.Series editor: Ostensen, Harald ISBN 92 4 154555 0 (NLM Classification: WE 141) The World Health Organization welcomes requests for permission to reproduce or translate its publications, in part or in full. Applications and enquiries should be addressed to the Office of Publications, World Health Organization, CH-1211 Geneva 27, Switzerland, which will be glad to provide the latest information on any changes made to the text, plans for new editions, and reprints and translations already available. © World Health Organization 2002 Publications of the World Health Organization enjoy copyright protection in accordance with the provisions of Protocol 2 of the Universal Copyright Convention. All rights reserved. -
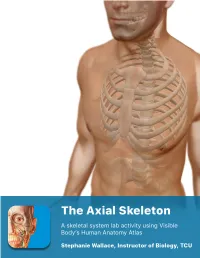
Lab Manual Axial Skeleton Atla
1 PRE-LAB EXERCISES When studying the skeletal system, the bones are often sorted into two broad categories: the axial skeleton and the appendicular skeleton. This lab focuses on the axial skeleton, which consists of the bones that form the axis of the body. The axial skeleton includes bones in the skull, vertebrae, and thoracic cage, as well as the auditory ossicles and hyoid bone. In addition to learning about all the bones of the axial skeleton, it is also important to identify some significant bone markings. Bone markings can have many shapes, including holes, round or sharp projections, and shallow or deep valleys, among others. These markings on the bones serve many purposes, including forming attachments to other bones or muscles and allowing passage of a blood vessel or nerve. It is helpful to understand the meanings of some of the more common bone marking terms. Before we get started, look up the definitions of these common bone marking terms: Canal: Condyle: Facet: Fissure: Foramen: (see Module 10.18 Foramina of Skull) Fossa: Margin: Process: Throughout this exercise, you will notice bold terms. This is meant to focus your attention on these important words. Make sure you pay attention to any bold words and know how to explain their definitions and/or where they are located. Use the following modules to guide your exploration of the axial skeleton. As you explore these bones in Visible Body’s app, also locate the bones and bone markings on any available charts, models, or specimens. You may also find it helpful to palpate bones on yourself or make drawings of the bones with the bone markings labeled. -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria.