WHO Manual of Diagnostic Imaging Radiographic Anatomy and Interpretation of the Musculoskeletal System
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

MARCH FRACTURE-PIED FORCE Developed Reactions to These Test Products and to Witte Peptone at Least As Severe As Those Shown by Our by Anaphylactic Case
FEB. 24, 1940 ANAPHYLAXIS AFTER TETANUS TOXOID MERCALTSORHL 95 erythema than similar dilutions of the extracts, and that within a few minutes of injection many persons MARCH FRACTURE-PIED FORCE developed reactions to these test products and to Witte peptone at least as severe as those shown by our BY anaphylactic case. Further investigations are in progress which it is hoped will be the subject of another paper. F. A. R. STAMMERS, Ch.M., F.R.C.S. Our observations throw considerable doubt on the value Major R.A.M.C., Surgical Specialist of scratch and intradermal tests as usually interpreted on the basis of early readings. Nevertheless, our patient Army medical officers everywhere have been asked to see was the only subject who developed mild anaphylactic all sorts of foot troubles precipitated by military training. symptoms-itching of the nose and tongue, slight swelling More often than not these are due to pre-existing con- of the lower lip, smarting of the eyes, and flushing of the ditions such as hallux rigidus, hallux valgus, hammer-toe, face-soon after 1 in 1,000 dilutions of Witte peptone and or pes planus, which, though formerly symptomless, break two other beef fibrin digests had been injected intra- down under the strain of route-marching, physical train- dermally; and she alone showed areas of redness, 50 to ing, and the general extra footwork the soldier- is called 60 mm. in diameter, swelling, and induration (" like upon to do in heavy army boots. Even a seemingly those occurring after staphylococcus toxoid ") about two normal foot may develop trouble under such circum- hours later at the sites of injection of these products. -

Original Article Pictorial Atlas of Symptomatic Accessory Ossicles by 18F-Sodium Fluoride (Naf) PET-CT
Am J Nucl Med Mol Imaging 2017;7(6):275-282 www.ajnmmi.us /ISSN:2160-8407/ajnmmi0069278 Original Article Pictorial atlas of symptomatic accessory ossicles by 18F-Sodium Fluoride (NaF) PET-CT Sharjeel Usmani1, Cherry Sit2, Gopinath Gnanasegaran2, Tim Van den Wyngaert3, Fahad Marafi4 1Department of Nuclear Medicine & PET/CT Imaging, Kuwait Cancer Control Center, Khaitan, Kuwait; 2Royal Free Hospital NHS Trust, London, UK; 3Antwerp University Hospital, Belgium; 4Jaber Al-Ahmad Molecular Imaging Center, Kuwait Received August 7, 2017; Accepted December 15, 2017; Epub December 20, 2017; Published December 30, 2017 Abstract: Accessory ossicles are developmental variants which are often asymptomatic. When incidentally picked up on imaging, they are often inconsequential and rarely a cause for concern. However, they may cause pain or discomfort due to trauma, altered stress, and over-activity. Nuclear scintigraphy may play a role in the diagnosis and localizing pain generators. 18F-Sodium Fluoride (NaF) is a PET imaging agent used in bone imaging. Although commonly used in imaging patients with cancer imaging malignancy, 18F-NaF may be useful in the evaluation of benign bone and joint conditions. In this article, we would like to present a spectrum of clinical cases and review the potential diagnostic utility of 18F-NaF in the assessment of symptomatic accessory ossicles in patients referred for staging cancers. Keywords: 18F-NaF PET/CT, accessory ossicles, hybrid imaging Introduction Accessory ossicles are developmental variants which are often asymptomatic. When inciden- Bone and joint pain is a common presentation tally picked up on imaging, they are often incon- in both primary and secondary practice. -

(Xgeva®) Related Osteonecrosis of the Jaw: a Retrospective Study
Journal of Clinical Medicine Article A Comparison of the Clinical and Radiological Extent of Denosumab (Xgeva®) Related Osteonecrosis of the Jaw: A Retrospective Study Zineb Assili 1, Gilles Dolivet 2, Julia Salleron 3 , Claire Griffaton-Tallandier 4, Claire Egloff-Juras 1 and Bérengère Phulpin 1,2,* 1 Faculty of Odontology, Lorraine University, 7 Avenue de la Forêt de Haye, 54505 Vandoeuvre les Nancy, France; [email protected] (Z.A.); [email protected] (C.E.-J.) 2 Department of Head and Neck and Dental Surgery, Institut de Cancérologie de Lorraine, 54519 Vadoeuvre-lès-Nancy, France; [email protected] 3 Cellule Data-Biostatistiques, Institut de Cancérologie de Lorraine, 54519 Vandoeuvre-lès-Nancy, France; [email protected] 4 Cabinet de Radiologie RX125, 125 Rue Saint-Dizier, 54000 Nancy, France; [email protected] * Correspondence: [email protected]; Tel.: +33-3-83-59-84-46 Abstract: Medication-related osteonecrosis of the jaw (MRONJ) is a severe side effect of antiresorptive medication. The aim of this study was to evaluate the incidence of denosumab-related osteonecrosis of the jaw and to compare the clinical and radiological extent of osteonecrosis. A retrospective study of patients who received Xgeva® at the Institut de Cancérologie de Lorraine (ICL) was performed. Patients for whom clinical and radiological (CBCT) data were available were divided into two groups: Citation: Assili, Z.; Dolivet, G.; “exposed” for patients with bone exposure and “fistula” when only a fistula through which the bone Salleron, J.; Griffaton-Tallandier, C.; could be probed was observed. The difference between clinical and radiological extent was assessed. -

Paleopathological Analysis of a Sub-Adult Allosaurus Fragilis (MOR
Paleopathological analysis of a sub-adult Allosaurus fragilis (MOR 693) from the Upper Jurassic Morrison Formation with multiple injuries and infections by Rebecca Rochelle Laws A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in Earth Sciences Montana State University © Copyright by Rebecca Rochelle Laws (1996) Abstract: A sub-adult Allosaurus fragilis (Museum of the Rockies specimen number 693 or MOR 693; "Big Al") with nineteen abnormal skeletal elements was discovered in 1991 in the Upper Jurassic Morrison Formation in Big Horn County, Wyoming at what became known as the "Big Al" site. This site is 300 meters northeast of the Howe Quarry, excavated in 1934 by Barnum Brown. The opisthotonic position of the allosaur indicated that rigor mortis occurred before burial. Although the skeleton was found within a fluvially-deposited sandstone, the presence of mud chips in the sandstone matrix and virtual completeness of the skeleton showed that the skeleton was not transported very far, if at all. The specific goals of this study are to: 1) provide a complete description and analysis of the abnormal bones of the sub-adult, male, A. fragilis, 2) develop a better understanding of how the bones of this allosaur reacted to infection and trauma, and 3) contribute to the pathological bone database so that future comparative studies are possible, and the hypothesis that certain abnormalities characterize taxa may be evaluated. The morphology of each of the 19 abnormal bones is described and each disfigurement is classified as to its cause: 5 trauma-induced; 2 infection-induced; 1 trauma- and infection-induced; 4 trauma-induced or aberrant, specific origin unknown; 4 aberrant; and 3 aberrant, specific origin unknown. -

Listen to the Associated Podcast Episodes: MSK: Fractures for the ABR Core Exam Parts 1-3, Available at Theradiologyreview.Com O
MSK: Fractures for Radiology Board Study, Matt Covington, MD Listen to the associated podcast episodes: MSK: Fractures for the ABR Core Exam Parts 1-3, available Listen to associated Podcast episodes: ABR Core Exam, Multisystemic Diseases Parts 1-3, available at at theradiologyreview.com or on your favorite podcast directory. Copyrighted. theradiologyreview.com or on your favorite podcast direcry. Fracture resulting From abnormal stress on normal bone = stress Fracture Fracture From normal stress on abnormal bone = insuFFiciency Fracture Scaphoid Fracture site with highest risk for avascular necrosis (proximal or distal)? Proximal pole scaphoid Fractures are at highest risk For AVN Comminuted Fracture at the base oF the First metacarpal = Rolando Fracture Non-comminuted Fracture at base oF the First metacarpal = Bennett Fracture The pull oF which tendon causes the dorsolateral dislocation in a Bennett fracture? The abductor pollicus longus tendon. Avulsion Fracture at the base oF the proximal phalanx with ulnar collateral ligament disruption = Gamekeeper’s thumb. Same Fracture but adductor tendon becomes caught in torn edge oF the ulnar collateral ligament? Stener’s lesion. IF Stener’s lesion is present this won’t heal on its own so you need surgery. You shouldn’t image a Gamekeeper’s thumb with stress views because you can convert it to a Stener’s lesion. Image with MRI instead. Distal radial Fracture with dorsal angulation = Colle’s Fracture (C to D= Colle’s is Dorsal) Distal radial Fracture with volar angulation = Smith’s Fracture (S -

Chronic Osteomyelitis of Jaw
Chronic Osteomyelitis of Jaw Dr. Dhawal Goyal, 1 Dr. Nilima Malik, 2 Dr. Neha Gupta, 3 Dr. Manoj Agarwal, 4 Dr. Rajani Kalla, 5 Dr. Sanyam Agarwal 6 1. Dr. Dhawal Goyal MDS, Oral Private Practitioner 2. Dr. Nilima Malik MDS Oral and Maxillofacial Surgery 3. Dr. Neha Gupta Assistant Professor, Dept. of Prosthodontics, RUHS College of Dental Sciences, Jaipur 4. Dr. Manoj Agarwal Assistant Professor, Dept. of Conservative Dentistry & Endodontics, RUHS College of Dental Sciences, Jaipur 5. Dr. Rajani Kalla Assistant Professor, Dept. of Prosthodontics, RUHS College of Dental Sciences, Jaipur 6. Dr. Sanyam Agarwal Medical Officer, Dept. of Conservative Dentistry & Endodontics, RUHS College of Dental Sciences, Jaipur The prevalence of osteomyelitis of jaws in third Cultures, bone biopsy, conventional radiography, world country is still at a higher rate despite newer scintigraphy, CT scan are used to diagnose chronic and powerful antibiotics and advances in dental osteomyelitis of jaws. Computed Tomograph helps care. This may be due to low socio-economical in determination of cortex and medullary status, unavailability of primary health care involvement of diseased bone better as compared to services, and poor nutritional status in the rural conventional radiograph. areas. Therapy for osteomyelitis of jaws requires a Osteomyelitis may be defined as an inflammatory multidisciplinary approach. A precise condition of the bone that usually begins as an microbiologic diagnosis and adequate debridement infection of the medullary cavity, rapidly involves of necrotic tissue are essential. Acute the Haversian system and quickly extends to hematogenous osteomyelitis usually responds to periosteum of the affected area. The infection then antimicrobial therapy. -

Little Bones the Sesamoids
Erica Chu 3/7/2014 Foot . Hallucal sesamoids . Lesser metatarsal sesamoids . Interphalangeal joint sesamoid of great toe . Os peroneum . Sesamoid within tibialis anterior tendon . Sesamoid within the posterior tibialis tendon Hand . Pollicis sesamoids . Second and fifth metacarpal sesamoids . Interphalangeal joint sesamoid of thumb . Pisiform Patella Fabella Small round or ovoid bones embedded in certain tendons Usually related to joint surfaces Osseous surfaces covered by cartilage Intimate with synovial- lined cavity Resnick D, Niwayama G, Feingold ML. The sesamoid bones of the hands and feet: participators in arthritis. Radiology 1977; 123:57- 62. Two types . Type A: Sesamoid located adjacent to articulation ▪ Patella ▪ Hallucis sesamoids ▪ Pollicis sesamoids . Type B: Bursa separates sesamoid from adjacent bone Type A Type B ▪ Sesamoid of peroneus longus Resnick D, Niwayama G, Feingold ML. The tendon sesamoid bones of the hands and feet: participators in arthritis. Radiology 1977; 123:57-62. Function . Protect tendons from damage . Increase efficiency or mechanical advantage of their associated muscle ▪ Part of gliding mechanism ▪ Modify pressure ▪ Decrease friction ▪ Alter muscle pull “in proportion as the pastern is oblique or slanting, two consequences will follow, less weight will be thrown on the pastern, and more on the sesamoid…and in that proportion concussion will be prevented.” Located in tendons that wrap around bony or fibrous pulleys . Peroneus longus tendon . Posterior tibialis tendon Adaptation to help maintain tendon structure . Resists compression or shear . Fibrous tissue ▪ Flexibility ▪ Toughness . Cartilaginous tissue ▪ Elasticity Can alter tendon appearance on MR Didolkar MM, Malone AL, Nunley JA, et al. Pseudotear of the peroneus longus tendon on MRI, secondary to a fibrocartilaginous node. -

Musculo-Skeletal System
Musculo-Skeletal System (Trunk, Limbs, and Head) somite: ectoderm dermatome General Statements: myotome Bilaterally, paraxial mesoderm become sclerotome neural crest somites and somitomeres. (Somitomeres develop ros- intermediate tral to the notochord in the head. They are like somites, but mesoderm neural tube smaller and less distinctly organized.) The mesoderm somatic mesoderm comprising each somite differentiates into three notochord regions: endoderm aorta — dermatome (lateral) which migrates to form dermis of the skin coelom — sclerotome (medial) forms most of the splanchnic mesoderm axial skeleton (vertebrae, ribs, and base of the skull). Mesoderm Regions — myotome (middle) forms skeletal mus- culature. Individual adult muscles are produced by merger of adjacent myotomes. Note: Nerves make early connections with adjacent myotomes and dermatomes, establishing a segmental innervation pattern. As myotome/dermatome cells migrate to assume adult positions, the segmental nerve supply must follow along to maintain its connection to the innervation target. (Recurrent laryngeal & phrenic nerves travel long distances because their targets migrated far away.) Skin. Consists of dermis and epidermis. Epidermis, including hair follicles & glands, is derived from ectoderm. Neural crest cells migrate into epidermis and become melanocytes. (Other neural crest cells become tactile disc receptors.) Dermis arises from mesoderm (dermatomes of somites). Each dermatome forms a continu- ous area of skin innervated by one spinal nerve. Because adjacent dermatomes overlap, a locus of adult skin is formed by 2 or 3 dermatomes, and innervated by 2 or 3 spinal nerves. Muscle. Muscles develop from mesoderm, except for muscles of the iris which arise from optic cup ectoderm. Cardiac and smooth muscles originate from splanchnic mesoderm. -
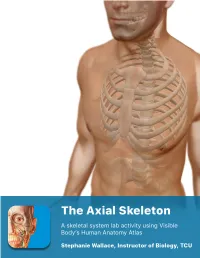
Lab Manual Axial Skeleton Atla
1 PRE-LAB EXERCISES When studying the skeletal system, the bones are often sorted into two broad categories: the axial skeleton and the appendicular skeleton. This lab focuses on the axial skeleton, which consists of the bones that form the axis of the body. The axial skeleton includes bones in the skull, vertebrae, and thoracic cage, as well as the auditory ossicles and hyoid bone. In addition to learning about all the bones of the axial skeleton, it is also important to identify some significant bone markings. Bone markings can have many shapes, including holes, round or sharp projections, and shallow or deep valleys, among others. These markings on the bones serve many purposes, including forming attachments to other bones or muscles and allowing passage of a blood vessel or nerve. It is helpful to understand the meanings of some of the more common bone marking terms. Before we get started, look up the definitions of these common bone marking terms: Canal: Condyle: Facet: Fissure: Foramen: (see Module 10.18 Foramina of Skull) Fossa: Margin: Process: Throughout this exercise, you will notice bold terms. This is meant to focus your attention on these important words. Make sure you pay attention to any bold words and know how to explain their definitions and/or where they are located. Use the following modules to guide your exploration of the axial skeleton. As you explore these bones in Visible Body’s app, also locate the bones and bone markings on any available charts, models, or specimens. You may also find it helpful to palpate bones on yourself or make drawings of the bones with the bone markings labeled. -

Parts of the Body 1) Head – Caput, Capitus 2) Skull- Cranium Cephalic- Toward the Skull Caudal- Toward the Tail Rostral- Toward the Nose 3) Collum (Pl
BIO 3330 Advanced Human Cadaver Anatomy Instructor: Dr. Jeff Simpson Department of Biology Metropolitan State College of Denver 1 PARTS OF THE BODY 1) HEAD – CAPUT, CAPITUS 2) SKULL- CRANIUM CEPHALIC- TOWARD THE SKULL CAUDAL- TOWARD THE TAIL ROSTRAL- TOWARD THE NOSE 3) COLLUM (PL. COLLI), CERVIX 4) TRUNK- THORAX, CHEST 5) ABDOMEN- AREA BETWEEN THE DIAPHRAGM AND THE HIP BONES 6) PELVIS- AREA BETWEEN OS COXAS EXTREMITIES -UPPER 1) SHOULDER GIRDLE - SCAPULA, CLAVICLE 2) BRACHIUM - ARM 3) ANTEBRACHIUM -FOREARM 4) CUBITAL FOSSA 6) METACARPALS 7) PHALANGES 2 Lower Extremities Pelvis Os Coxae (2) Inominant Bones Sacrum Coccyx Terms of Position and Direction Anatomical Position Body Erect, head, eyes and toes facing forward. Limbs at side, palms facing forward Anterior-ventral Posterior-dorsal Superficial Deep Internal/external Vertical & horizontal- refer to the body in the standing position Lateral/ medial Superior/inferior Ipsilateral Contralateral Planes of the Body Median-cuts the body into left and right halves Sagittal- parallel to median Frontal (Coronal)- divides the body into front and back halves 3 Horizontal(transverse)- cuts the body into upper and lower portions Positions of the Body Proximal Distal Limbs Radial Ulnar Tibial Fibular Foot Dorsum Plantar Hallicus HAND Dorsum- back of hand Palmar (volar)- palm side Pollicus Index finger Middle finger Ring finger Pinky finger TERMS OF MOVEMENT 1) FLEXION: DECREASE ANGLE BETWEEN TWO BONES OF A JOINT 2) EXTENSION: INCREASE ANGLE BETWEEN TWO BONES OF A JOINT 3) ADDUCTION: TOWARDS MIDLINE -

Osteochondrosis – Primary Epiphyseal (Articular/Subchondral) Lesion Can Heal Or Can Progress
60 120 180 1 distal humeral condyles 2 medial epicondyle 3 proximal radial epiphysis 4 anconeal process Lab Ret study N=1018 . Normal . Affected . Total 688 (67.6%) . Total 330 (32.4%) . Male 230 (62.2%) . Male 140 (37.8%) . Female 458 (70.7%) . Female 190 (29.3%) Affected dogs N=330 1affected site - 250 (75.7%) 2 affected sites - 68 (20.6%) 3 affected sites - 12 (3.6%) immature skeletal diseases denis novak technique for skeletal radiography tissue < 12 cm “non-grid” (“table-top”) technique “high detail” system radiation safety diagnosis X – rays examination Ultrasound CT bilateral lesions - clinical signs ? unilateral present > one type of lesion 2ry arthrosis Common Osteochondrosis – primary epiphyseal (articular/subchondral) lesion can heal or can progress Osteochondritis dissecans – free articular fragment will progress Arthrosis Osteochondrosis talus / tarsus Lumbosacral OCD Lumbosacral OCD Inflammatory diseases Panosteitis – non infectious Hypertrophic osteodystrophy (HOD) – perhaps infectious Osteomyelitis - infectious Panosteitis New medullary bone Polyostotic Multiple lesions in one bone Symmetrical or nonsymmetrical Sclerotic pattern B I L A T E R A L periosteal new bone forms with chronicity Cross sections of a tibia different locations Hypertrophic osteodystrophy (HOD) Dogs are systemically ill, febrile, anorectic, reluctant to walk most will recover Radiographic changes of HOD . Polyostotic . Metaphyseal . Symmetrical . Changes of lesion Early Mid Late lytic “plates” in acute case HOD - 4 m ret – lesions are present -

Rheumatology-TP 1..2
Rheumatology 2015 The British Society for Rheumatology and British Health Professionals in Rheumatology Annual Meeting 2015 28 April – 30 April 2015 Manchester Central, Manchester, UK The abstracts are freely available online to all visitors to the Rheumatology website (http://www.rheumatology.oxfordjournals.org). RheumatologyDOI is incorrect10.1093/rheumatology/ker000ker000ContentsRheumatologyContents- Contents2012000000002012Volume 51 Supplement 3 May 2012 Volume 54 Supplement 1 April 2015 CONTENTS Rheumatology 2015 Abstracts INVITED SPEAKER ABSTRACTS (TUESDAY 28 APRIL 2015) I01–I03 Imaging in rheumatology: a practical perspective i1 I04–I06 Biologics in SLE: getting close to lift off (at last!) i1 I07–I09 Shared decision making and self-management support: why they matter in i1 rheumatology I10–I11 Challenges of remote and rural rheumatology i2 I12–I15 A step in the right direction: addressing foot health in rheumatoid arthritis i2 I16–I18 Musculoskeletal health and vocational rehabilitation i3 I19–I21 Making it happen: optimizing the service to RA patients i4 I22–I24 Community-based physical activity for osteoarthritis: the emerging role of i4 non-healthcare professionals I25–I26 Post-doctoral, PhD and postgraduate student network i5 I27–I32 Jewels in the Crown and top scoring abstracts (including Michael Mason and Garrod i5 prize winners and young investigator award) I33 Heberden Round i6 INVITED SPEAKER ABSTRACTS (WEDNESDAY 29 APRIL 2015) I34–I36 Pain in the 21st century: sensory–immune interactions, biologic agents and i8 bisphosphonates