Pathogens on Japanese Quince (Chaenomeles Japonica) Plants
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Botanical Gardens in France
France Total no. of Botanic Gardens recorded in France: 104, plus 10 in French Overseas Territories (French Guiana, Guadeloupe, Martinique and Réunion). Approx. no. of living plant accessions recorded in these botanic gardens: c.300,000 Approx. no. of taxa in these collections: 30,000 to 40,000 (20,000 to 25,000 spp.) Estimated % of pre-CBD collections: 80% to 90% Notes: In 1998 36 botanic gardens in France issued an Index Seminum. Most were sent internationally to between 200 and 1,000 other institutions. Location: ANDUZE Founded: 1850 Garden Name: La Bambouseraie (Maurice Negre Parc Exotique de Prafrance) Address: GENERARGUES, F-30140 ANDUZE Status: Private. Herbarium: Unknown. Ex situ Collections: World renowned collection of more than 100 species and varieties of bamboos grown in a 6 ha plot, including 59 spp.of Phyllostachys. Azaleas. No. of taxa: 260 taxa Rare & Endangered plants: bamboos. Special Conservation Collections: bamboos. Location: ANGERS Founded: 1895 Garden Name: Jardin Botanique de la Faculté de Pharmacie Address: Faculte Mixte de Medecine et Pharmacie, 16 Boulevard Daviers, F-49045 ANGERS. Status: Universiy Herbarium: No Ex situ Collections: Trees and shrubs (315 taxa), plants used for phytotherapy and other useful spp. (175 taxa), systematic plant collection (2,000 taxa), aromatic, perfume and spice plants (22 spp), greenhouse plants (250 spp.). No. of taxa: 2,700 Rare & Endangered plants: Unknown Location: ANGERS Founded: 1863 Garden Name: Arboretum Gaston Allard Address: Service des Espaces Verts de la Ville, Mairie d'Angers, BP 3527, 49035 ANGERS Cedex. Situated: 9, rue du Château d’Orgement 49000 ANGERS Status: Municipal Herbarium: Yes Approx. -

Garden Mastery Tips March 2006 from Clark County Master Gardeners
Garden Mastery Tips March 2006 from Clark County Master Gardeners Flowering Quince Flowering quince is a group of three hardy, deciduous shrubs: Chaenomeles cathayensis, Chaenomeles japonica, and Chaenomeles speciosa. Native to eastern Asia, flowering quince is related to the orchard quince (Cydonia oblonga), which is grown for its edible fruit, and the Chinese quince (Pseudocydonia sinensis). Flowering quince is often referred to as Japanese quince (this name correctly refers only to C. japonica). Japonica is often used regardless of species, and flowering quince is still called Japonica by gardeners all over the world. The most commonly cultivated are the hybrid C. superba and C. speciosa, not C. japonica. Popular cultivars include ‘Texas Scarlet,’ a 3-foot-tall plant with red blooms; ‘Cameo,’ a double, pinkish shrub to five feet tall; and ‘Jet Trail,’ a white shrub to 3 feet tall. Flowering quince is hardy to USDA Zone 4 and is a popular ornamental shrub in both Europe and North America. It is grown primarily for its bright flowers, which may be red, pink, orange, or white. The flowers are 1 to 2 inches in diameter, with five petals, and bloom in late winter or early spring. The glossy dark green leaves appear soon after flowering and turn yellow or red in autumn. The edible quince fruit is yellowish-green with reddish blush and speckled with small dots. The fruit is 2 to 4 inches in diameter, fragrant, and ripens in fall. The Good The beautiful blossoms of flowering quince Flowering quince is an easy-to-grow, drought-tolerant shrub that does well in shady spots as well as sun (although more sunlight will produce better flowers). -

Chaenomeles Speciosa) in the Naxi and Tibetan Highlands of NW Yunnan, China
Cultural and Ecosystem Services of Flowering Quince (Chaenomeles speciosa) in the Naxi and Tibetan Highlands of NW Yunnan, China. Authors: Lixin Yang, Selena Ahmed, John Richard Stepp, Yanqinag Zhao, Ma Jun Zeng, Shengji Pei, Dayuan Xue, and Gang Xu The final publication is available at Springer via https://dx.doi.org/10.1007/s12231-015-9318-7. Yang, Lixin, Selena Ahmed, John Richard Stepp, Yanqinag Zhao, Ma Jun Zeng, Shengji Pei, Dayuan Xue, and Gang Xu. “Cultural Uses, Ecosystem Services, and Nutrient Profile of Flowering Quince (Chaenomeles Speciosa) in the Highlands of Western Yunnan, China.” Economic Botany 69, no. 3 (September 2015): 273–283. doi:10.1007/s12231-015-9318-7. Made available through Montana State University’s ScholarWorks scholarworks.montana.edu Cultural Uses, Ecosystem Services, and Nutrient Profile Chaenomeles speciosa of Flowering Quince ( ) in the Highlands 1 of Western Yunnan, China 2,3 3,4 ,3,5 6 LIXIN YANG ,SELENA AHMED ,JOHN RICHARD STEPP* ,YANQINAG ZHAO , 7 2 ,3 2 MA JUN ZENG ,SHENGJI PEI ,DAYUAN XUE* , AND GANG XU 2State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institutes of Botany, Chinese Academy of Sciences, Kunming, China 3College of Life and Environmental Science, Minzu University of China, Beijing, China 4Department of Health and Human Development, Montana State University, Bozeman, MT, USA 5Department of Anthropology, University of Florida, Gainesville, FL, USA 6College of Forestry and Vocational Technology in Yunnan, Kunming, China 7Southwest Forestry University, Bailongshi, Kunming, China *Corresponding author; e-mail: [email protected]; [email protected] Introduction ample light but is tolerant of partial shade. -

List of the Import Prohibited Plants
List of the Import Prohibited Plants The Annexed Table 2 of the amended Enforcement Ordinance of the Plant Protection Law (Amended portions are under lined) Districts Prohibited Plants Quarantine Pests 1. Yemen, Israel, Saudi Arabia, Fresh fruits of akee, avocado, star berry, Mediterranean fruit fly Syria, Turkey, Jordan, Lebanon, allspice, olive, cashew nut, kiwi fruit, Thevetia (Ceratitis capitata) Albania, Italy, United Kingdom peruviana, carambola, pomegranate, jaboticaba, (Great Britain and Northern broad bean, alexandrian laurel, date palm, Ireland, hereinafter referred to as Muntingia calabura, feijoa, pawpaw, mammee "United Kingdom"), Austria, apple, longan, litchi, and plants of the genera Netherlands, Cyprus, Greece, Ficus, Phaseolus, Diospyros(excluding those Croatia, Kosovo, Switzerland, listed in appendix 41), Carissa, Juglans, Morus, Spain, Slovenia, Serbia, Germany, Coccoloba, Coffea, Ribes, Vaccinium, Hungary, France, Belgium, Passiflora, Dovyalis, Ziziphus, Spondias, Musa Bosnia and Herzegovina, (excluding immature banana), Carica (excluding Portugal, Former Yugoslav those listed in appendix 1), Psidium, Artocarpus, Republic of Macedonia, Malta, , Annona, Malpighia, Santalum, Garcinia, Vitis Montenegro, Africa, Bermuda, (excluding those listed in appendices 3 and 54), Argentina, Uruguay, Ecuador, El Eugenia, Mangifera (excluding those listed in Salvador, Guatemala, Costa Rica, appendices 2 ,36 ,43 ,51 and 53), Ilex, Colombia, Nicaragua, West Indies Terminalia and Gossypium, and Plants of the (excluding Cuba, Dominican family Sapotaceae, Cucurbitaceae (excluding Republic,Puerto Rico), Panama, those listed in appendices 3 and 42), Cactaceae Paraguay, Brazil, Venezuela, (excluding those listed in appendix 35), Peru, Bolivia, Honduras, Australia Solanaceae (excluding those listed in (excluding Tasmania), Hawaiian appendices 3 and 42), Rosaceae (excluding Islands those listed in appendices 3 and 31) and Rutaceae (excluding those listed in appendices 4 to 8 ,39 ,45 and 56). -

Phylogeny of Maleae (Rosaceae) Based on Multiple Chloroplast Regions: Implications to Genera Circumscription
Hindawi BioMed Research International Volume 2018, Article ID 7627191, 10 pages https://doi.org/10.1155/2018/7627191 Research Article Phylogeny of Maleae (Rosaceae) Based on Multiple Chloroplast Regions: Implications to Genera Circumscription Jiahui Sun ,1,2 Shuo Shi ,1,2,3 Jinlu Li,1,4 Jing Yu,1 Ling Wang,4 Xueying Yang,5 Ling Guo ,6 and Shiliang Zhou 1,2 1 State Key Laboratory of Systematic and Evolutionary Botany, Institute of Botany, Chinese Academy of Sciences, Beijing 100093, China 2University of the Chinese Academy of Sciences, Beijing 100043, China 3College of Life Science, Hebei Normal University, Shijiazhuang 050024, China 4Te Department of Landscape Architecture, Northeast Forestry University, Harbin 150040, China 5Key Laboratory of Forensic Genetics, Institute of Forensic Science, Ministry of Public Security, Beijing 100038, China 6Beijing Botanical Garden, Beijing 100093, China Correspondence should be addressed to Ling Guo; [email protected] and Shiliang Zhou; [email protected] Received 21 September 2017; Revised 11 December 2017; Accepted 2 January 2018; Published 19 March 2018 Academic Editor: Fengjie Sun Copyright © 2018 Jiahui Sun et al. Tis is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Maleae consists of economically and ecologically important plants. However, there are considerable disputes on generic circumscription due to the lack of a reliable phylogeny at generic level. In this study, molecular phylogeny of 35 generally accepted genera in Maleae is established using 15 chloroplast regions. Gillenia isthemostbasalcladeofMaleae,followedbyKageneckia + Lindleya, Vauquelinia, and a typical radiation clade, the core Maleae, suggesting that the proposal of four subtribes is reasonable. -
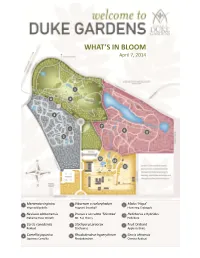
What's in Bloom
WHAT’S IN BLOOM April 7, 2014 5 4 6 2 7 1 9 8 3 12 10 11 1 Mertensia virginica 5 Viburnum x carlcephalum 9 Malus ‘Hopa’ Virginia Bluebells Fragrant Snowball Flowering Crabapple 2 Neviusia alabamensis 6 Prunus x serrulata ‘Shirotae’ 10 Helleborus x hybridus Alabama Snow Wreath Mt. Fuji Cherry Hellebore 3 Cercis canadensis 7 Stachyurus praecox 11 Fruit Orchard Redbud Stachyurus Apple cultivars 4 Camellia japonica 8 Rhododendron hyperythrum 12 Cercis chinensis Japanese Camellia Rhododendron Chinese Redbud WHAT’S IN BLOOM April 7, 2014 BLOMQUIST GARDEN OF NATIVE PLANTS Amelanchier arborea Common Serviceberry Sanguinaria canadensis Bloodroot Cornus florida Flowering Dogwood Stylophorum diphyllum Celandine Poppy Thalictrum thalictroides Rue Anemone Fothergilla major Fothergilla Trillium decipiens Chattahoochee River Trillium Hepatica nobilis Hepatica Trillium grandiflorum White Trillium Hexastylis virginica Wild Ginger Hexastylis minor Wild Ginger Trillium pusillum Dwarf Wakerobin Illicium floridanum Florida Anise Tree Trillium stamineum Blue Ridge Wakerobin Malus coronaria Sweet Crabapple Uvularia sessilifolia Sessileleaf Bellwort Mertensia virginica Virginia Bluebells Pachysandra procumbens Allegheny spurge Prunus americana American Plum DORIS DUKE CENTER GARDENS Camellia japonica Japanese Camellia Pulmonaria ‘Diana Clare’ Lungwort Cercis canadensis Redbud Prunus persica Flowering Peach Puschkinia scilloides Striped Squill Cercis chinensis Redbud Sanguinaria canadensis Bloodroot Clematis armandii Evergreen Clematis Spiraea prunifolia Bridalwreath -

Comitetul De Redacţie
Analele Ştiinţifice ale Universităţii „Al. I. Cuza” Iaşi, s. Biologie animală, Tom LIII, 2007 LEAF-MINING INSECTS ENCOUNTERED IN THE FOREST RESERVE OF HÂRBOANCA, VASLUI COUNTY Alina-Maria STOLNICU “Alexandru Ioan Cuza” University, Iasi, the Faculty of Biology, Carol I Blvd., no. 22, 700505, Iaşi, Romania e-mail: [email protected] Abstract. As a result of a series of studies conducted within the Forest Reserve of Hârboanca (Vaslui) between June 2005 and October 2006, there were identified 60 species of leaf-mining insects, belonging to 14 families, from three different orders: Lepidoptera (83%), Diptera (12%) and Hymenoptera (5%). The “mines” caused by the larvae of these insects were identified on 34 different species of hosting plants, mostly wooden plants. The leaf-mining Lepidoptera and Hymenoptera larvae are more likely to grow on wooden plants, while those belonging to the Diptera order prefer herbaceous plants. One of the species, Phyllonorycter issikii (Kumata) found here was signaled for the first time in Romanian fauna, while other ten species were encountered for the first time in Moldavia. Keywords: leaf-mining insects, Forest Reserve of Hârboanca, Romanian, fauna. Rezumat. Insecte miniere semnalate în Rezervaţia Forestieră Hârboanca (Vaslui). În urma studiilor efectuate în Rezervaţia Forestieră Hârboanca (Vaslui) în perioada iunie 2005 - octombrie 2006 s-au identificat 60 de specii de insecte miniere care aparţin la 14 familii, grupate în 3 ordine: Lepidoptera (83%), Diptera (12%) şi Hymenoptera (5%). Minele provocate de larvele insectelor miniere au fost identificate pe 34 de specii de plante-gazdă, majoritatea fiind de esenţă lemnoasă. Larvele lepidopterelor şi himenopterelor miniere se dezvoltă mai mult pe plantele lemnoase, în schimb dipterele preferă plantele ierboase. -

Chaenomeles Spp. - Flowering Quince (Rosaceae) ------Chaenomeles Is a Functional Flowering Hedge, Border, Twigs Or Specimen Shrub That Can Be Used Near Buildings
Chaenomeles spp. - Flowering Quince (Rosaceae) ----------------------------------------------------------------------------------- Chaenomeles is a functional flowering hedge, border, Twigs or specimen shrub that can be used near buildings. -buds small and reddish in color The major appeal of Flowering Quince is its showy -lightly armed (terminal and axillary spines) but brief flowering period. The rest of the year it’s a -young bark is reddish and cherry-like utilitarian thorny shrub with limited aesthetic Trunk attributes. -gray brown -many small diameter stems closely crowded, arising FEATURES from the ground Form -large shrubs USAGE 2-6' tall Function -vased shaped -sun tolerant, long-lived shrub habit with -useful as a hedge or barrier many small Texture diameter -medium in foliage and when bare stems Assets -1:1 height to -urban tolerant width ratio -withstand severe pruning -rapid growth -drought tolerant Culture -early spring flowers -full sun -dense growth and long-lived -adaptable to a wide range of soil conditions -lightly armed for effective "crowd control" -thrives under stressful conditions Liabilities -moderate availability -poor autumn color Foliage -trash can accumulate among its many small diameter -alternate, lanceolate stems (maintenance headache) -serrate margins -prone to cosmetic damage by insects -somewhat leathery -sheds foliage in summer in response to drought or -to 4" long disease pressure -leafy, kidney-shaped stipules (an ID feature) Habitat -summer color is dense medium green and attractive, -Zones 4 to 8, depending on species new growth often bronze -Native to the Orient (China, Japan) -autumn color yellowish green SELECTIONS Alternates -urban tolerant shrub with vase-shaped winter form (e.g. Berberis thunbergii, Berberis x mentorensis, Spiraea nipponica 'Snowmound' etc.) -early spring flowering shrubs (e.g. -

Management of Japanese Quince (Chaenomeles Japonica) Orchards
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Epsilon Open Archive Management of Japanese Quince (Chaenomeles japonica) Orchards Management of Japanese Quince (Chaenomeles japonica) Orchards D. Kviklysa*, S. Ruisab, K. Rumpunenc aLithuanian Institute of Horticulture, Babtai, Lithuania b Dobele Horticultural Plant Breeding Experimental Station, Dobele, Latvia cBalsgård–Department of Horticultural Plant Breeding, Swedish University of Agricultural Sciences, Kristianstad, Sweden *Correspondence to [email protected] SUMMARY In this paper, advice for establishment and management of Japanese quince (Chaenomeles japonica) orchards is summarised. Japanese quince is a minor fruit crop in Latvia and Lithuania, currently being developed by plant breeding research. Preferences for site and soil are discussed and recommendations for planting and field management are proposed. INTRODUCTION Among the four known Chaenomeles species native to China, Tibet and Japan, Japanese quince (Chaenomeles japonica) is the species best adapted to the North European climate and it has been intro- duced as a minor fruit crop in Latvia and Lithuania (Rumpunen 2002, Tiits 1989, Tics 1992). At present, we are aware of only one active plant breeding programme that is aimed at improving Japanese quince as a fruit crop. This programme is being jointly conducted by the Department of Plant Biology, Helsinki University, Finland; Dobele Horticultural Plant Breeding Experimental Station, Latvia; the Lithuanian Institute of Horticulture, Lithuania and Balsgård–Department of Horticultural Plant Breeding, Swedish University of Agricultural Sciences, Sweden (Rumpunen 2002). As a first step to improve Japanese quince, phenotypic selection has taken place in orchards in Latvia and Lithuania. Superior selections have been cloned and planted in comparative field trials in Finland, Italy, Latvia, Lithuania and Sweden. -

Collections Policy
Chicago Botanic Garden COLLECTIONS POLICY 1 Collections Policy July 2018 2 COLLECTIONS POLICY TABLE OF CONTENTS Mission Statement ................................................................................................................... 1 Intent of Collections Policy Document ..................................................................................... 1 Purpose of Collections .............................................................................................................. 1 Scope of Collections ................................................................................................................. 1 1) Display Plant Collections .......................................................................................... 2 Seasonal Display Collections ........................................................................... 2 Permanent Display Gardens ............................................................................ 2 Aquatic Garden ................................................................................... 2 Bonsai Collection ................................................................................. 3 Graham Bulb Garden .......................................................................... 3 Grunsfeld Children’s Growing Garden ................................................. 3 Circle Garden ....................................................................................... 3 Kleinman Family Cove ........................................................................ -

Detección De Leucoptera Sinuella (Reutti) (Lepidoptera: Lyonetiidae) En Chile, Con La Identificación De Algunos Parasitoides Asociados
www.biotaxa.org/rce. ISSN 0718-8994 (online) Revista Chilena de Entomología (2019) 45 (1): 65-77. Artículo Científico Detección de Leucoptera sinuella (Reutti) (Lepidoptera: Lyonetiidae) en Chile, con la identificación de algunos parasitoides asociados Detection of Leucoptera sinuella (Reutti) (Lepidoptera: Lyonetiidae) in Chile, with the identification of some associated parasitoids Ariel Sandoval C.1, Sandra Ide M.1, Sergio Rothmann T.2, Evelyn Zúñiga S.3, Paula Bosch E.3 y Max Peragallo R.4 1Servicio Agrícola y Ganadero, División Protección Agrícola y Forestal, Departamento Sanidad Vegetal, Subdepartamento Vigilancia y Control de Plagas Forestales, Santiago, CHILE. E-mail: [email protected] 2Servicio Agrícola y Ganadero, Subdepartamento de Laboratorios y Estación Cuarentenaria Agrícola, Unidad de Entomología, Santiago, CHILE. 3Servicio Agrícola y Ganadero, Región Metropolitana, División Protección Agrícola y Forestal, Santiago, CHILE. 4Servicio Agrícola y Ganadero, Región de O’Higgins, División Protección Agrícola y Forestal, Santiago, CHILE. ZooBank: urn:lsid:zoobank.org:pub:26793211-8ADF-46A4-AE97-72D78E8CC322 Resumen. En marzo del 2015 el Servicio Agrícola y Ganadero (SAG), a través de actividades de vigilancia forestal, detectó por primera vez en Chile la presencia de Leucoptera sinuella (Reutti), atacando follaje de álamos (Populus spp., Salicaceae), en la comuna de Talagante (Región Metropolitana de Santiago). Actividades de prospección desarrolladas por el SAG han determinado que este microlepidóptero se encuentra distribuido en diversas comunas de las regiones de Valparaíso, Metropolitana de Santiago, Libertador General Bernardo O’Higgins, Maule, Ñuble y Biobío. Adicionalmente, fueron identificadas siete especies de microhimenópteros parasitoides asociados aL. sinuella, pertenecientes a las familias Eulophidae (6 especies) y Chalcididae (1 especie). -

Chaenomeles (Flowering Quince)
EARLY SPRING COLOR TO Chaenomeles speciosa ‘Scarlet Storm’ WARM YOUR SOUL Photo: Kathy Barrowclough John Frett Flowering quince has been cultivated for thousands of years in China, Korea and Japan as a bonsai specimen and for use in flower arrangements. A member of the rose family, it was first introduced into English gardens in the late 1700’s and found its way into gardens in the United States in the early to mid 1800’s. It was a favorite in rural gardens and on farms for its attractive flowers, edible fruit and cover for compact, growing 4–6 feet tall with a slightly wider spread so wildlife. Its popularity is rejuvenative pruning is optional. Stems can be spined or spine- evidenced by the more than less. Fruit is intermediate in size but still bitter/tart if not al- 500 cultivars described. lowed to fully ripen. Breeding has primarily focused on this group, with flower size, number and color range maximized. There are three species commonly grown in gardens: Chinese quince (Pseudocydonia sinensis) is the largest of Chaenomeles speciosa, the plants that we offer. It is a large shrub or small tree common flowering quince; C. growing 10–25 feet tall. The upright growth habit can be easily japonica, Japanese flowering trained into a tree form to display the colorful, exfoliating bark, quince; and the hybrid Chaenomeles speciosa ‘Toyo Nishiki’ which occurs in shades of grey, green and orange brown. species C. × superba, a cross Photo: Rick Darke Stems often exhibit fluted or sinuous growth. Branches lack between the previous two spines.