Egr3-Dependent Muscle Spindle Stretch Receptor Intrafusal Muscle Fiber Differentiation and Fusimotor Innervation Homeostasis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

VIEW Open Access Muscle Spindle Function in Healthy and Diseased Muscle Stephan Kröger* and Bridgette Watkins
Kröger and Watkins Skeletal Muscle (2021) 11:3 https://doi.org/10.1186/s13395-020-00258-x REVIEW Open Access Muscle spindle function in healthy and diseased muscle Stephan Kröger* and Bridgette Watkins Abstract Almost every muscle contains muscle spindles. These delicate sensory receptors inform the central nervous system (CNS) about changes in the length of individual muscles and the speed of stretching. With this information, the CNS computes the position and movement of our extremities in space, which is a requirement for motor control, for maintaining posture and for a stable gait. Many neuromuscular diseases affect muscle spindle function contributing, among others, to an unstable gait, frequent falls and ataxic behavior in the affected patients. Nevertheless, muscle spindles are usually ignored during examination and analysis of muscle function and when designing therapeutic strategies for neuromuscular diseases. This review summarizes the development and function of muscle spindles and the changes observed under pathological conditions, in particular in the various forms of muscular dystrophies. Keywords: Mechanotransduction, Sensory physiology, Proprioception, Neuromuscular diseases, Intrafusal fibers, Muscular dystrophy In its original sense, the term proprioception refers to development of head control and walking, an early im- sensory information arising in our own musculoskeletal pairment of fine motor skills, sensory ataxia with un- system itself [1–4]. Proprioceptive information informs steady gait, increased stride-to-stride variability in force us about the contractile state and movement of muscles, and step length, an inability to maintain balance with about muscle force, heaviness, stiffness, viscosity and ef- eyes closed (Romberg’s sign), a severely reduced ability fort and, thus, is required for any coordinated move- to identify the direction of joint movements, and an ab- ment, normal gait and for the maintenance of a stable sence of tendon reflexes [6–12]. -

Ultrastructural Cardiac Muscle and Cardiac Microvasculature Changes in Experimental Murine Infections Acta Scientiae Veterinariae, Vol
Acta Scientiae Veterinariae ISSN: 1678-0345 [email protected] Universidade Federal do Rio Grande do Sul Brasil Tejero, Felix; Arias-Mota, Lourdes Lorena; Roschman-González, Antonio; Aso, Pedro María; Finol, Héctor José Trypanosoma evansi: Ultrastructural Cardiac Muscle and Cardiac Microvasculature Changes in Experimental Murine Infections Acta Scientiae Veterinariae, vol. 38, núm. 3, 2010, pp. 279-285 Universidade Federal do Rio Grande do Sul Porto Alegre, Brasil Available in: http://www.redalyc.org/articulo.oa?id=289021902008 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Acta Scientiae Veterinariae. 38(3): 279-285, 2010. ORIGINAL ARTICLE ISSN 1679-9216 (Online) Pub. 910 Trypanosoma evansi: Ultrastructural Cardiac Muscle and Cardiac Microvasculature Changes in Experimental Murine Infections* Felix Tejero1, Lourdes Lorena Arias-Mota1, Antonio Roschman-González2, Pedro María Aso3 & Héctor José Finol2 ABSTRACT Background: Trypanosoma evansi is the etiologic agent of the equine trypanosomosis, a disease related to the detriment of the extensive bovine farming in the Venezuelan grasslands. Even though macroscopic pathologies such as anemia, pale mucosa, icteric tissues, generalized edema, splenomegaly, liver and renal hypertrophy, abortion, anoestrus, emaciation, lymphadenopathies, striated muscle atrophy as well as epicardiac and endocardiac hemorrhages have been described for infections with the agent, no reports of any heart ultrastructural change in experimental or natural infections induced by Venezuelan T. evansi isolates are available. So, a transmission electron microscopic approach to the problem was needed. This work describes cell features of the cardiac myocyte and the cardiac microvasculature ultrastructure in mice experimentally infected with an equine local isolate of T. -

Multifactorial Erβ and NOTCH1 Control of Squamous Differentiation and Cancer
Multifactorial ERβ and NOTCH1 control of squamous differentiation and cancer Yang Sui Brooks, … , Karine Lefort, G. Paolo Dotto J Clin Invest. 2014;124(5):2260-2276. https://doi.org/10.1172/JCI72718. Research Article Oncology Downmodulation or loss-of-function mutations of the gene encoding NOTCH1 are associated with dysfunctional squamous cell differentiation and development of squamous cell carcinoma (SCC) in skin and internal organs. While NOTCH1 receptor activation has been well characterized, little is known about how NOTCH1 gene transcription is regulated. Using bioinformatics and functional screening approaches, we identified several regulators of the NOTCH1 gene in keratinocytes, with the transcription factors DLX5 and EGR3 and estrogen receptor β (ERβ) directly controlling its expression in differentiation. DLX5 and ERG3 are required for RNA polymerase II (PolII) recruitment to the NOTCH1 locus, while ERβ controls NOTCH1 transcription through RNA PolII pause release. Expression of several identified NOTCH1 regulators, including ERβ, is frequently compromised in skin, head and neck, and lung SCCs and SCC-derived cell lines. Furthermore, a keratinocyte ERβ–dependent program of gene expression is subverted in SCCs from various body sites, and there are consistent differences in mutation and gene-expression signatures of head and neck and lung SCCs in female versus male patients. Experimentally increased ERβ expression or treatment with ERβ agonists inhibited proliferation of SCC cells and promoted NOTCH1 expression and squamous differentiation both in vitro and in mouse xenotransplants. Our data identify a link between transcriptional control of NOTCH1 expression and the estrogen response in keratinocytes, with implications for differentiation therapy of squamous cancer. Find the latest version: https://jci.me/72718/pdf Research article Multifactorial ERβ and NOTCH1 control of squamous differentiation and cancer Yang Sui Brooks,1,2 Paola Ostano,3 Seung-Hee Jo,1,2 Jun Dai,1,2 Spiro Getsios,4 Piotr Dziunycz,5 Günther F.L. -

Supplementary Materials
Supplementary Materials: Supplemental Table 1 Abbreviations FMDV Foot and Mouth Disease Virus FMD Foot and Mouth Disease NC Non-treated Control DEGs Differentially Expressed Genes RNA-seq High-throughput Sequencing of Mrna RT-qPCR Quantitative Real-time Reverse Transcriptase PCR TCID50 50% Tissue Culture Infective Doses CPE Cytopathic Effect MOI Multiplicity of Infection DMEM Dulbecco's Modified Eagle Medium FBS Fetal Bovine Serum PBS Phosphate Buffer Saline QC Quality Control FPKM Fragments per Kilo bases per Million fragments method GO Gene Ontology KEGG Kyoto Encyclopedia of Genes and Genomes R Pearson Correlation Coefficient NFKBIA NF-kappa-B Inhibitor alpha IL6 Interleukin 6 CCL4 C-C motif Chemokine 4 CXCL2 C-X-C motif Chemokine 2 TNF Tumor Necrosis Factor VEGFA Vascular Endothelial Growth Gactor A CCL20 C-C motif Chemokine 20 CSF2 Macrophage Colony-Stimulating Factor 2 GADD45B Growth Arrest and DNA Damage Inducible 45 beta MYC Myc proto-oncogene protein FOS Proto-oncogene c-Fos MCL1 Induced myeloid leukemia cell differentiation protein Mcl-1 MAP3K14 Mitogen-activated protein kinase kinase kinase 14 IRF1 Interferon regulatory factor 1 CCL5 C-C motif chemokine 5 ZBTB3 Zinc finger and BTB domain containing 3 OTX1 Orthodenticle homeobox 1 TXNIP Thioredoxin-interacting protein ZNF180 Znc Finger Protein 180 ZNF36 Znc Finger Protein 36 ZNF182 Zinc finger protein 182 GINS3 GINS complex subunit 3 KLF15 Kruppel-like factor 15 Supplemental Table 2 Primers for Verification of RNA-seq-detected DEGs with RT-qPCR TNF F: CGACTCAGTGCCGAGATCAA R: -

Interpretation of Sensory Information from Skeletal Muscle Receptors for External Control Milan Djilas
Interpretation of Sensory Information From Skeletal Muscle Receptors For External Control Milan Djilas To cite this version: Milan Djilas. Interpretation of Sensory Information From Skeletal Muscle Receptors For External Control. Automatic. Université Montpellier II - Sciences et Techniques du Languedoc, 2008. English. tel-00333530 HAL Id: tel-00333530 https://tel.archives-ouvertes.fr/tel-00333530 Submitted on 23 Oct 2008 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. UNIVERSITE MONTPELLIER II SCIENCES ET TECHNIQUES DU LANGUEDOC T H E S E pour obtenir le grade de DOCTEUR DE L'UNIVERSITE MONTPELLIER II Formation doctorale: SYSTEMES AUTOMATIQUES ET MICROELECTRONIQUES Ecole Doctorale: INFORMATION, STRUCTURES ET SYSTEMES présentée et soutenue publiquement par Milan DJILAS le 13 octobre 2008 Titre: INTERPRETATION DES INFORMATIONS SENSORIELLES DES RECEPTEURS DU MUSCLE SQUELETTIQUE POUR LE CONTROLE EXTERNE INTERPRETATION OF SENSORY INFORMATION FROM SKELETAL MUSCLE RECEPTORS FOR EXTERNAL CONTROL JURY Jacques LEVY VEHEL Directeur de Recherches, INRIA Rapporteur -

Genome-Wide DNA Methylation Analysis of KRAS Mutant Cell Lines Ben Yi Tew1,5, Joel K
www.nature.com/scientificreports OPEN Genome-wide DNA methylation analysis of KRAS mutant cell lines Ben Yi Tew1,5, Joel K. Durand2,5, Kirsten L. Bryant2, Tikvah K. Hayes2, Sen Peng3, Nhan L. Tran4, Gerald C. Gooden1, David N. Buckley1, Channing J. Der2, Albert S. Baldwin2 ✉ & Bodour Salhia1 ✉ Oncogenic RAS mutations are associated with DNA methylation changes that alter gene expression to drive cancer. Recent studies suggest that DNA methylation changes may be stochastic in nature, while other groups propose distinct signaling pathways responsible for aberrant methylation. Better understanding of DNA methylation events associated with oncogenic KRAS expression could enhance therapeutic approaches. Here we analyzed the basal CpG methylation of 11 KRAS-mutant and dependent pancreatic cancer cell lines and observed strikingly similar methylation patterns. KRAS knockdown resulted in unique methylation changes with limited overlap between each cell line. In KRAS-mutant Pa16C pancreatic cancer cells, while KRAS knockdown resulted in over 8,000 diferentially methylated (DM) CpGs, treatment with the ERK1/2-selective inhibitor SCH772984 showed less than 40 DM CpGs, suggesting that ERK is not a broadly active driver of KRAS-associated DNA methylation. KRAS G12V overexpression in an isogenic lung model reveals >50,600 DM CpGs compared to non-transformed controls. In lung and pancreatic cells, gene ontology analyses of DM promoters show an enrichment for genes involved in diferentiation and development. Taken all together, KRAS-mediated DNA methylation are stochastic and independent of canonical downstream efector signaling. These epigenetically altered genes associated with KRAS expression could represent potential therapeutic targets in KRAS-driven cancer. Activating KRAS mutations can be found in nearly 25 percent of all cancers1. -

A Web-Platform for Analysis of Host Factors Involved in Viral Infections Discovered by Genome Wide Rnai Screen
Electronic Supplementary Material (ESI) for Molecular BioSystems. This journal is © The Royal Society of Chemistry 2017 vhfRNAi: A web-platform for analysis of host factors involved in viral infections discovered by genome wide RNAi screen Anamika Thakur#, Abid Qureshi# and Manoj Kumar* Bioinformatics Centre, Institute of Microbial Technology, Council of Scientific and Industrial Research, Sector 39A, Chandigarh-160036, India #Equal contribution * To whom correspondence should be addressed. Tel, 91-172-6665453; Fax, 91-172- 2690585; 91-172-2690632; Email, [email protected] Supplementary Tables Table S1: Statistics of unique and duplicate host factors in each virus Table S2: Table denoting genes common among different viruses Table S3: Statistics of GWAS analysis Table S1. Statistics of unique and duplicate host factors in each virus S. No. Virus Unique-Entries Duplicate-Entries 1. Adeno-associated virus (AAV) 926 533 2. Avian influenza virus (AIV) 0 11 3. Borna disease virus (BDV) 14 20 4. Dengue virus 2 (DEN-2) 27 13 5. Hepatitis C virus (HCV) 236 213 6. Human immunodeficiency virus 1 (HIV) 1388 857 7. Human parainfluenza virus 3 (HPIV-3) 0 27 8. Human herpesvirus 1 (HSV-1) 34 38 9. Influenza A virus (IAV) 700 513 10. Lymphocytic choriomeningitis virus (LCMV) 0 54 11. Marburgvirus (MARV) 0 11 12. Poliovirus (PV) 3340 1035 13. Rotavirus (RV) 347 175 14. Sendai virus (SeV) 32 27 15. Sindbis virus (SIV) 70 41 16. Vaccinia virus (VACV) 482 296 17. Vesicular stomatitis virus (VSV) 9 78 18. West Nile virus (WNV) 313 137 Table S1. Statistics of unique host factors for each virus having overlapping and unique factors in different viruses Non-overlap – Overall- S. -

(7E) Powerpoint Lecture Outline Chapter 8: Control of Movement
Carlson (7e) PowerPoint Lecture Outline Chapter 8: Control of Movement This multimedia product and its contents are protected under copyright law. The following are prohibited by law: •any public performance or display, including transmission of any image over a network; •preparation of any derivative work, including extraction, in whole or in part, of any images; •any rental, lease, or lending of the program. Copyright 2001 by Allyn & Bacon Skeletal Muscle n Movements of our body are accomplished by contraction of the skeletal muscles l Flexion: contraction of a flexor muscle draws in a limb l Extension: contraction of extensor muscle n Skeletal muscle fibers have a striated appearance n Skeletal muscle is composed of two fiber types: l Extrafusal: innervated by alpha-motoneurons from the spinal cord: exert force l Intrafusal: sensory fibers that detect stretch of the muscle u Afferent fibers: report length of intrafusal: when stretched, the fibers stimulate the alpha-neuron that innervates the muscle fiber: maintains muscle tone u Efferent fibers: contraction adjusts sensitivity of afferent fibers. 8.2 Copyright 2001 by Allyn & Bacon Skeletal Muscle Anatomy n Each muscle fiber consists of a bundle of myofibrils l Each myofibril is made up of overlapping strands of actin and myosin l During a muscle twitch, the myosin filaments move relative to the actin filaments, thereby shortening the muscle fiber 8.3 Copyright 2001 by Allyn & Bacon Neuromuscular Junction n The neuromuscular junction is the synapse formed between an alpha motor neuron -

Tissue Engineered Myelination and the Stretch Reflex Arc Sensory Circuit: Defined Medium Ormulation,F Interface Design and Microfabrication
University of Central Florida STARS Electronic Theses and Dissertations, 2004-2019 2009 Tissue Engineered Myelination And The Stretch Reflex Arc Sensory Circuit: Defined Medium ormulation,F Interface Design And Microfabrication John Rumsey University of Central Florida Part of the Biology Commons Find similar works at: https://stars.library.ucf.edu/etd University of Central Florida Libraries http://library.ucf.edu This Doctoral Dissertation (Open Access) is brought to you for free and open access by STARS. It has been accepted for inclusion in Electronic Theses and Dissertations, 2004-2019 by an authorized administrator of STARS. For more information, please contact [email protected]. STARS Citation Rumsey, John, "Tissue Engineered Myelination And The Stretch Reflex Arc Sensory Circuit: Defined Medium Formulation, Interface Design And Microfabrication" (2009). Electronic Theses and Dissertations, 2004-2019. 3826. https://stars.library.ucf.edu/etd/3826 TISSUE ENGINEERED MYELINATION AND THE STRETCH REFLEX ARC SENSORY CIRCUIT: DEFINED MEDIUM FORMULATION, INTERFACE DESIGN AND MICROFABRICATION by JOHN WAYNE RUMSEY B.S. University of Florida, 2001 M.S. University of Central Florida, 2004 A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Burnett School of Biomedical Sciences in the College of Medicine at the University of Central Florida Orlando, Florida Fall Term 2009 Major Professor: James J. Hickman ABSTRACT The overall focus of this research project was to develop an in vitro tissue- engineered system that accurately reproduced the physiology of the sensory elements of the stretch reflex arc as well as engineer the myelination of neurons in the systems. In order to achieve this goal we hypothesized that myelinating culture systems, intrafusal muscle fibers and the sensory circuit of the stretch reflex arc could be bioengineered using serum-free medium formulations, growth substrate interface design and microfabrication technology. -

Identification of Hair Shaft Progenitors That Create a Niche for Hair Pigmentation
Downloaded from genesdev.cshlp.org on September 27, 2021 - Published by Cold Spring Harbor Laboratory Press Identification of hair shaft progenitors that create a niche for hair pigmentation Chung-Ping Liao,1 Reid C. Booker,1 Sean J. Morrison,2,3,4,5,6 and Lu Q. Le1,4,5 1Department of Dermatology, 2Department of Pediatrics, 3Children’s Research Institute, 4Simmons Comprehensive Cancer Center, 5Hamon Center for Regenerative Science and Medicine, 6Howard Hughes Medical Institute, University of Texas Southwestern Medical Center, Dallas, Texas 75390, USA Hair differentiates from follicle stem cells through progenitor cells in the matrix. In contrast to stem cells in the bulge, the identities of the progenitors and the mechanisms by which they regulate hair shaft components are poorly understood. Hair is also pigmented by melanocytes in the follicle. However, the niche that regulates follicular melanocytes is not well characterized. Here, we report the identification of hair shaft progenitors in the matrix that are differentiated from follicular epithelial cells expressing transcription factor KROX20. Depletion of Krox20 lin- eage cells results in arrest of hair growth, confirming the critical role of KROX20+ cells as antecedents of structural cells found in hair. Expression of stem cell factor (SCF) by these cells is necessary for the maintenance of differen- tiated melanocytes and for hair pigmentation. Our findings reveal the identities of hair matrix progenitors that regulate hair growth and pigmentation, partly by creating an SCF-dependent niche for follicular melanocytes. [Keywords: stem cell factor (SCF); hair pigmentation; hair shaft progenitor cell; hair follicle stem cell; hair matrix; KROX20] Supplemental material is available for this article. -
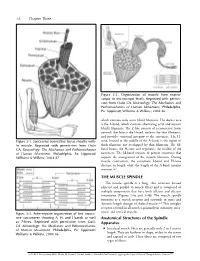
THE MUSCLE SPINDLE Anatomical Structures of the Spindle Apparatus
56 Chapter Three Figure 3-2. Organization of muscle from macro- scopic to microscopic levels. Reprinted with permis- sion from Oatis CA. Kinesiology: The Mechanics and Pathomechanics of Human Movement. Philadelphia, Pa: Lippincott Williams & Wilkins; 2004:46. which contains only actin (thin) filaments. The darker area is the A-band, which contains alternating actin and myosin (thick) filaments. The Z-line consists of a connective tissue network that bisects the I-band, anchors the thin filaments, and provides structural integrity to the sarcomere. The H- Figure 3-1. Successive connective tissue sheaths with- zone, located in the middle of the A-band, is the region of in muscle. Reprinted with permission from Oatis thick filaments not overlapped by thin filaments. The M- CA. Kinesiology: The Mechanics and Pathomechanics band bisects the H-zone and represents the middle of the of Human Movement. Philadelphia, Pa: Lippincott sarcomere. The M-band consists of protein structures that Williams & Wilkins; 2004:47. support the arrangement of the myosin filaments. During muscle contraction, the sarcomere I-band and H-zone decrease in length while the length of the A-band remains constant.2,3 THE MUSCLE SPINDLE The muscle spindle is a long, thin structure located adjacent and parallel to muscle fibers and is composed of multiple components that have both afferent and efferent innervation (Figures 3-4a and 3-4b). The muscle spindle functions as a stretch receptor and responds to static and dynamic length changes of skeletal muscle.4-6 This complex receptor is found in all muscles, primarily in extremity, inter- costal, and cervical muscles. -

Be Able to Describe a Stretch Reflex
be able to describe a stretch reflex .(1) (2) Define muscle tone (3) be able to explain what is muscle tone (4) describe the structure , innervations and function of the muscle spindle . (5) explain what is meant by static and dynamic stretch reflex . (6) describe the spinal and supraspinal regulation of the stretch reflex . (7) describe the inverse stretch reflex and its function Stretch reflex: Whenever a muscle is stretched suddenly, excitation of the muscle spindle causes reflex contraction of the large muscle fiber (See the pictures). It is deep and monosynaptic reflex. Stretch response is produced by co-activation of alpha & gamma motor neurons. But it is maintained mainly by the tonic ( continuous) discharge of Gamma Efferent neurons. Muscle spindle: It is a sensory receptors which are distributed throughout the muscle and it sends information to nervous system about muscle length or rate of change in muscle length. - Each spindle is built around 3-12 intrafusal muscle fibers. - Muscle spindles are parallel to extrafusal muscle fibers and they are attached to them or to the tendon. Types of Intrafusal fibers Each intrafusal fiber consists of: (1) Central non-contractile area (receptor 2) Nuclear chain fibers: area). thinner and shorter 1) Nuclear bag fibers: than nuclear bag fibers (2) Peripheral contractile area. contain many nuclei in , and have one line of a dilated central area ( “ nuclei spread in a chain bag ” ) . Typically there along the receptor area are 2 nuclear bag fibers . There are 4 – 9 per spindle . nuclear chain