Source Apportionment of Inorganic Solutes in Surface Waters of Lake Baikal Watershed
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
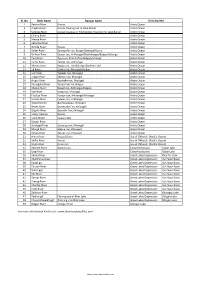
List of Rivers of Mongolia
Sl. No River Name Russian Name Draining Into 1 Yenisei River Russia Arctic Ocean 2 Angara River Russia, flowing out of Lake Baikal Arctic Ocean 3 Selenge River Сэлэнгэ мөрөн in Sükhbaatar, flowing into Lake Baikal Arctic Ocean 4 Chikoy River Arctic Ocean 5 Menza River Arctic Ocean 6 Katantsa River Arctic Ocean 7 Dzhida River Russia Arctic Ocean 8 Zelter River Зэлтэрийн гол, Bulgan/Selenge/Russia Arctic Ocean 9 Orkhon River Орхон гол, Arkhangai/Övörkhangai/Bulgan/Selenge Arctic Ocean 10 Tuul River Туул гол, Khentii/Töv/Bulgan/Selenge Arctic Ocean 11 Tamir River Тамир гол, Arkhangai Arctic Ocean 12 Kharaa River Хараа гол, Töv/Selenge/Darkhan-Uul Arctic Ocean 13 Eg River Эгийн гол, Khövsgöl/Bulgan Arctic Ocean 14 Üür River Үүрийн гол, Khövsgöl Arctic Ocean 15 Uilgan River Уйлган гол, Khövsgöl Arctic Ocean 16 Arigiin River Аригийн гол, Khövsgöl Arctic Ocean 17 Tarvagatai River Тарвагтай гол, Bulgan Arctic Ocean 18 Khanui River Хануй гол, Arkhangai/Bulgan Arctic Ocean 19 Ider River Идэр гол, Khövsgöl Arctic Ocean 20 Chuluut River Чулуут гол, Arkhangai/Khövsgöl Arctic Ocean 21 Suman River Суман гол, Arkhangai Arctic Ocean 22 Delgermörön Дэлгэрмөрөн, Khövsgöl Arctic Ocean 23 Beltes River Бэлтэсийн Гол, Khövsgöl Arctic Ocean 24 Bügsiin River Бүгсийн Гол, Khövsgöl Arctic Ocean 25 Lesser Yenisei Russia Arctic Ocean 26 Kyzyl-Khem Кызыл-Хем Arctic Ocean 27 Büsein River Arctic Ocean 28 Shishged River Шишгэд гол, Khövsgöl Arctic Ocean 29 Sharga River Шарга гол, Khövsgöl Arctic Ocean 30 Tengis River Тэнгис гол, Khövsgöl Arctic Ocean 31 Amur River Russia/China -

Научное Обозрение. Педагогические Науки № 1 Scientific Review
РОССИЙСКАЯ АКАДЕМИЯ ЕСТЕСТВОЗНАНИЯ ИЗДАТЕЛЬСКИЙ ДОМ «АКАДЕМИЯ ЕСТЕСТВОЗНАНИЯ» THE RUSSIAN ACADEMY OF NATURAL HISTORY PUBLISHING HOUSE «ACADEMY OF NATURAL HISTORY» НАУЧНОЕ ОБОЗРЕНИЕ. ПЕДАГОГИЧЕСКИЕ НАУКИ № 1 SCIENTIFIC REVIEW. PEDAGOGICAL SCIENCES 2015 Учредитель: Издательский дом Журнал «НАУЧНОЕ ОБОЗРЕНИЕ» выходил с 1894 «Академия Естествознания», по 1903 год в издательстве П.П. Сойкина. Главным 440026, Россия, г. Пенза, редактором журнала был Михаил Михайлович ул. Лермонтова, д. 3 Филиппов. В журнале публиковались работы Ленина, Плеханова, Циолковского, Менделеева, Бехтерева, Founding: Лесгафта и др. Publishing House «Academy Of Natural History» Journal «Scientific Review» published from 1894 to 440026, Russia, Penza, 1903. P.P. Soykin was the publisher. Mikhail Filippov 3 Lermontova str. was the Editor in Chief. The journal published works of Lenin, Plekhanov, Tsiolkovsky, Mendeleev, Адрес редакции Bekhterev, Lesgaft etc. 440026, Россия, г. Пенза, ул. Лермонтова, д. 3 Тел. +7 (499) 704-1341 Факс +7 (8452) 477-677 e-mail: [email protected] Edition address 440026, Russia, Penza, 3 Lermontova str. Tel. +7 (499) 704-1341 Fax +7 (8452) 477-677 e-mail: [email protected] Подписано в печать 01.03.2015 Формат 60х90 1/8 Типография ИД Издательский дом М.М. Филиппов (M.M. Philippov) «Академия Естествознания, 440026, Россия, г. Пенза, С 2014 года издание журнала возобновлено ул. Лермонтова, д. 3 Академией Естествознания Signed in print 01.03.2015 From 2014 edition of the journal resumed by Format 60x90 8.1 Academy of Natural History Typography Главный редактор: М.Ю. Ледванов Publishing House «Academy Of Natural History» Editor in Chief: M.Yu. Ledvanov 440026, Russia, Penza, 3 Lermontova str. Редакционная коллегия (Editorial Board) А.Н. Курзанов (A.N. -

In the Lands of the Romanovs: an Annotated Bibliography of First-Hand English-Language Accounts of the Russian Empire
ANTHONY CROSS In the Lands of the Romanovs An Annotated Bibliography of First-hand English-language Accounts of The Russian Empire (1613-1917) OpenBook Publishers To access digital resources including: blog posts videos online appendices and to purchase copies of this book in: hardback paperback ebook editions Go to: https://www.openbookpublishers.com/product/268 Open Book Publishers is a non-profit independent initiative. We rely on sales and donations to continue publishing high-quality academic works. In the Lands of the Romanovs An Annotated Bibliography of First-hand English-language Accounts of the Russian Empire (1613-1917) Anthony Cross http://www.openbookpublishers.com © 2014 Anthony Cross The text of this book is licensed under a Creative Commons Attribution 4.0 International license (CC BY 4.0). This license allows you to share, copy, distribute and transmit the text; to adapt it and to make commercial use of it providing that attribution is made to the author (but not in any way that suggests that he endorses you or your use of the work). Attribution should include the following information: Cross, Anthony, In the Land of the Romanovs: An Annotated Bibliography of First-hand English-language Accounts of the Russian Empire (1613-1917), Cambridge, UK: Open Book Publishers, 2014. http://dx.doi.org/10.11647/ OBP.0042 Please see the list of illustrations for attribution relating to individual images. Every effort has been made to identify and contact copyright holders and any omissions or errors will be corrected if notification is made to the publisher. As for the rights of the images from Wikimedia Commons, please refer to the Wikimedia website (for each image, the link to the relevant page can be found in the list of illustrations). -

Subject of the Russian Federation)
How to use the Atlas The Atlas has two map sections The Main Section shows the location of Russia’s intact forest landscapes. The Thematic Section shows their tree species composition in two different ways. The legend is placed at the beginning of each set of maps. If you are looking for an area near a town or village Go to the Index on page 153 and find the alphabetical list of settlements by English name. The Cyrillic name is also given along with the map page number and coordinates (latitude and longitude) where it can be found. Capitals of regions and districts (raiony) are listed along with many other settlements, but only in the vicinity of intact forest landscapes. The reader should not expect to see a city like Moscow listed. Villages that are insufficiently known or very small are not listed and appear on the map only as nameless dots. If you are looking for an administrative region Go to the Index on page 185 and find the list of administrative regions. The numbers refer to the map on the inside back cover. Having found the region on this map, the reader will know which index map to use to search further. If you are looking for the big picture Go to the overview map on page 35. This map shows all of Russia’s Intact Forest Landscapes, along with the borders and Roman numerals of the five index maps. If you are looking for a certain part of Russia Find the appropriate index map. These show the borders of the detailed maps for different parts of the country. -

Baikal Project 2012-2014 Results and Events Booklet.Pdf
Photo by Elena Chumak GEF: “The GEF unites 182 countries in partnership with international institutions, non-governmental organizations (NGOs), and the private sector to address global environmental issues while supporting national sustainable development initiatives. Today the GEF is the largest public funder of projects to improve the global environment. An independently operating financial organization, the GEF provides grants for projects related to biodiversity, climate change, international waters, land degradation, the ozone layer, and persistent organic pollutants. Since 1991, GEF has achieved a strong track record with developing countries and countries with economies in transition, providing $9.2 billion in grants and leveraging $40 billion in co-financing for over 2,700 projects in over 168 countries. www.thegef.org” UNDP: “UNDP partners with people at all levels of society to help build nations that can withstand crisis, and drive and sustain the kind of growth that improves the quality of life for everyone. On the ground in 177 countries and territories, we offer global perspective and local insight to help empower lives and build resilient nations. www.undp.org” UNOPS: is an operational arm of the United Nations, helping a range of partners implement $1 billion worth of aid and development projects every year. UNOPS mission is to expand the capacity of the UN system and its partners to implement peacebuilding, humanitarian and development operations that matter for people in need. Photo by Elena Chumak Contents Project Achievements -

River Water Quality of the Selenga-Baikal Basin: Part II—Metal Partitioning Under Different Hydroclimatic Conditions
water Article River Water Quality of the Selenga-Baikal Basin: Part II—Metal Partitioning under Different Hydroclimatic Conditions Nikolay Kasimov 1, Galina Shinkareva 1,* , Mikhail Lychagin 1, Sergey Chalov 1 , Margarita Pashkina 1, Josefin Thorslund 2 and Jerker Jarsjö 2 1 Faculty of Geography, Lomonosov Moscow State University, 119991 Moscow, Russia; [email protected] (N.K.); [email protected] (M.L.); [email protected] (S.C.); [email protected] (M.P.) 2 Department of Physical Geography and the Bolin Centre for Climate Research, Stockholm University, SE-106 91 Stockholm, Sweden; josefi[email protected] (J.T.); [email protected] (J.J.) * Correspondence: [email protected]; Tel.: +7-909-633-2239 Received: 4 July 2020; Accepted: 20 August 2020; Published: 26 August 2020 Abstract: The partitioning of metals and metalloids between their dissolved and suspended forms in river systems largely governs their mobility and bioavailability. However, most of the existing knowledge about catchment-scale metal partitioning in river systems is based on a limited number of observation points, which is not sufficient to characterize the complexity of large river systems. Here we present an extensive field-based dataset, composed of multi-year data from over 100 monitoring locations distributed over the large, transboundary Selenga River basin (of Russia and Mongolia), sampled during different hydrological seasons. The aim is to investigate on the basin scale, the influence of different hydroclimatic conditions on metal partitioning and transport. Our results showed that the investigated metals exhibited a wide range of different behaviors. Some metals were mostly found in the dissolved form (84–96% of Mo, U, B, and Sb on an average), whereas many others predominantly existed in suspension (66–87% of Al, Fe, Mn, Pb, Co, and Bi). -

Editorial Features Travel News Equipment Books
THE FACTS AND VIEWPOINTS IN THIS SECTION ARE NOT NECESSARILY THE VIEWS OF X-RAY MAG. ITEMS PRESENTED IN THIS SECTION HAS NOT BEEN TESTED BY X- Bottom Crawlers RAY MAG STAFF, NOR ARE THE ITEMS WARRANTED, INFORMATION PROVIDED IS CONDENSED FROM MANUFACTURERS DESCRIPTIONS. TEXTS ARE USUALLY EDITED 100% cotton Tribal Shark Men’s T FOR LENGTH, CLARITY AND STYLE LINKS ACTIVE AT THE TIME OF PUBLICATIONS Bold oriental brush strokes on mocha t-shirt. POINT & CLICK Price: US$16.95 www.bottomcrawlers.com ON BOLD LINKS Edited by Gunild Pak Symes ALL PHOTOS COURTESY OF THE MANUFACTURERS OR VENDORS Spring Dive Fashion Stylin’ Togs for Your Tank Stylish Jewelry from the Sea Have a little fun with your gear and A piece of dyed mother of pearl or shell is cut and set by hand in each of these get ‘em grinning with this polyethylene air tank stainless steel pendants. Other designs by the Canadian based husband-and-wife shirt inspired by Lifesavers hard candies. design team Eric Jean-Louis and Vivian Cheng come in bamboo, coconut, coral, Price: US$21.97 www.divingtoysfromdivetonight.com ox bone, steel, Mah Jong and Washi styles. Price: US$75.00 Blendcreations.com Patagonia beach foot- wear fad: Cloud Walker for WaterGirls. O2 Rainwear Full-grain leather upper, Blue or Yellow Lightwieght, breath- pigskin lining and suede Jacket, $29.95 able, waterproof cover footbed. Five colors. Price: Pant, $20.99 for sea bound divers US$80.00 www.patagonia.com O2rainwear.com Sea Inspired Style The fashion label Kawayan (Philippino for ’bamboo’) was created by two sisters, Anna Frances and Anna Mae Dioso. -

Gene Pool of Buryats: Clinal Variability and Territorial Subdivision Based on Data of Y�Chromosome Markers V
ISSN 10227954, Russian Journal of Genetics, 2014, Vol. 50, No. 2, pp. 180–190. © Pleiades Publishing, Inc., 2014. Original Russian Text © V.N. Kharkov, K.V. Khamina, O.F. Medvedeva, K.V. Simonova, E.R. Eremina, V.A. Stepanov, 2014, published in Genetika, 2014, Vol. 50, No. 2, pp. 203–213. HUMAN GENETICS Gene Pool of Buryats: Clinal Variability and Territorial Subdivision Based on Data of YChromosome Markers V. N. Kharkova, K. V. Khaminaa, O. F. Medvedevaa, K. V. Simonovaa, E. R. Ereminab, and V. A. Stepanova a Institute of Medical Genetics, Siberian Branch, Russian Academy of Medical Sciences, nab. Ushaiki 10, Tomsk, 634050 Russia email: [email protected], vladimir.kharkov@medgenetics b Department of Therapy, Buryat State University, ul. Smolin 24a, UlanUde, 670000 Russia Received April 23, 2013 Abstract—The structure of the Buryat gene pool has been studied based on the composition and frequency of Ychromosome haplogroups in eight geographically distant populations. Eleven haplogroups have been found in the Buryat gene pool, two of which are the most frequent (N1c1 and C3d). The greatest difference in haplogroup frequencies was fixed between western and eastern Buryat samples. The evaluation of genetic diversity based on haplogroup frequencies revealed that it has low values in most of the samples. The evalua tion of the genetic differentiation of the examined samples using an analysis of molecular variance (AMOVA) shows that the Buryat gene pool is highly differentiated by haplotype frequencies. Phylogenetic analysis within haplogroups N1c1 and C3d revealed a strong founder effect, i.e., reduced diversity and starlike phy logeny of the median network of haplotypes that form specific subclusters. -

Epistolary Encounters: Diary and Letter Pastiche in Neo-Victorian Fiction
Epistolary Encounters: Diary and Letter Pastiche in Neo-Victorian Fiction By Kym Michelle Brindle Thesis submitted in fulfilment for the degree of PhD in English Literature Department of English and Creative Writing Lancaster University September 2010 ProQuest Number: 11003475 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a com plete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. uest ProQuest 11003475 Published by ProQuest LLC(2018). Copyright of the Dissertation is held by the Author. All rights reserved. This work is protected against unauthorized copying under Title 17, United States C ode Microform Edition © ProQuest LLC. ProQuest LLC. 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106- 1346 Abstract This thesis examines the significance of a ubiquitous presence of fictional letters and diaries in neo-Victorian fiction. It investigates how intercalated documents fashion pastiche narrative structures to organise conflicting viewpoints invoked in diaries, letters, and other addressed accounts as epistolary forms. This study concentrates on the strategic ways that writers put fragmented and found material traces in order to emphasise such traces of the past as fragmentary, incomplete, and contradictory. Interpolated documents evoke ideas of privacy, confession, secrecy, sincerity, and seduction only to be exploited and subverted as writers idiosyncratically manipulate epistolary devices to support metacritical agendas. Underpinning this thesis is the premise that much literary neo-Victorian fiction is bound in an incestuous relationship with Victorian studies. -

155 the Formation of Impact Territories the Basins Of
L.G.Namzhilova., Journal of agricultural sciences №11 (02):155-158, 2013 155 THE FORMATION OF IMPACT TERRITORIES THE BASINS OF TRANSBOUNDARY RIVER SELENGA L.G. Namzhilova Baikal Institute of Nature Management SB RAS (BNM SB RAS), Ulan-Ude, Russia ABSTRACT We consider the impact territories of a regional system of nature management, which are centers of environmental stress in the Selenga River basin. Their studies involve assessing the pollution of natural environments and aims to develop measures to minimize their impact on the environment that will promote a regional system of ecological optimization of nature (RSPP), in general. KEY WORDS: Formation, impact territory, Selenge river basin INTRODUCTION We consider the impact territories of a regional system of nature management, which are centers of environmental stress in the Selenga River basin. Their studies involve assessing the pollution of natural environments and aims to develop measures to minimize their impact on the environment that will promote a regional system of ecological optimization of nature (RSPP), in general. METHODS AND MATERIAL Analysis of the national experience of nature territorial-industrial complex, which as a result of management study (NM) shows that the study of the negative anthropogenous impact have occurred functioning of the subsystems of the RSNM [3] negative changes in the environment that led to the indicating the nature management conflict, as well as emergence and development of critical ecological complex of ecological problems will provide in the situations. For the first time there were allocated to studied territory to distinguish impact territories the North of Russia. Impacted areas in the RSNM are (areas), which are characterized by a number of sources of ecological stress, and their emergence and natural and ecological and socio-economic indicators expansion is one of the most important contemporary for ecological optimization of the RSNM. -

Overview: Recent Advances on the Understanding of the Northern
https://doi.org/10.5194/acp-2021-341 Preprint. Discussion started: 19 May 2021 c Author(s) 2021. CC BY 4.0 License. 1 1 Overview: Recent advances on the understanding of the Northern Eurasian environments and of the 2 urban air quality in China - Pan Eurasian Experiment (PEEX) program perspective 3 4 Hanna K. Lappalainen1,2, Tuukka Petäjä1,2, Timo Vihma3, Jouni Räisänen1, Alexander Baklanov4, Sergey 5 Chalov5, Igor Esau6, Ekaterina Ezhova1, Matti Leppäranta1 , Dmitry Pozdnyakov7, Jukka Pumpanen 8, Meinrat 6 O. Andreae9,42,43, Mikhail Arshinov10, Eija Asmi3, Jianhui Bai11, Igor Bashmachnikov7, Boris Belan10, Federico 7 Bianchi1, Boris Biskaborn12, Michael Boy1, Jaana Bäck13, Bin Cheng3, Natalia Ye Chubarova5, Jonathan 8 Duplissy1, Egor Dyukarev14, Konstantinos Eleftheriadis15, Martin Forsius16, Martin Heimann17, Sirkku 9 Juhola20, Vladimir Konovalov18, Igor Konovalov19, Pavel Konstantinov5,33, Kajar Koster13, Elena Lapsina 21, 10 Anna Lintunen1,13, Alexander Mahura1, Risto Makkonen3, Svetlana Malkhazova5, Ivan Mammarella1, Stefano 11 Mammola22,23, Stephany Mazon1, Outi Meinander3, Eugene Mikhailov24, 25, Victoria Miles6, Stanislav 12 Myslenko5, Dmitry Orlov5, Jean-Daniel Paris26, Roberta Pirazzini3, Olga Popovicheva27, Jouni Pulliainen3, 13 Kimmo Rautiainen3, Torsten Sachs28 , Vladimir Shevchenko29, Andrey Skorokhod30, Andreas Stohl31, Elli 14 Suhonen1, Erik S. Thomson 32, Marina Tsidilina39, Veli-Pekka Tynkkynen 34, Petteri Uotila1, Aki Virkkula3, 35 6 36 37 37 38 15 Nadezhda Voropay , Tobias Wolf , Sayaka Yasunaka , Jiahua Zhang , Yubao -

1962-1963 Undergraduate Catalogue
FOUNDED 1791 • BURLINGTON, VERMONT Bulletin of THE UNIVERSITY OF VERMONT THE CATALOGUE • 1962-1963 ANNOUNCEMENTS • 1963-1 964 Cover photo: The Guy V. Bailey Library completed in 1961. Photo by Eldred. Inside back cover: Entrance to Bailey Library. Photo by Eldred. Cover design by John MacLeod. Printed by Queen City Printers Inc. CORRESPONDENCE Admissions Requests for a catalogue, or information concerning admission poli cies and procedures, rooms and tuition Undergraduate Colleges Director of Admissions College of Medicine Dean of the College of Medicine Graduate College Dean of the Graduate College Evening Division Director of Evening Division Summer Session Director of the Summer Session Conferences and Institutes Director of Conferences and Institutes Transcripts of Records Office of Admissions and Records Scholarships and Loans Director of Financial Aid Employment of Seniors and Alumni Director of Placement Matters of Alumni Interest Alumni Secretary Matters of General University Interest The President Bulletin of the University of Vermont VOLUME 60 APRIL, 1963 NUMBER 13 Published by the University of Vermont, Burlington, Vermont, sixteen times a year—once each in December, February, April, May, June, and August; twice in September and November; and three times in January and March. Second-class postage paid at Burlington, Vermont. THE CONTENTS PERSONNEL 1 INTRODUCTION 24 STUDENT LIFE 3 J THE ADMISSION OF STUDENTS 44 STUDENT EXPENSES 47 GENERAL INFORMATION S2 THE COLLEGE OF AGRICULTURE 37 THE COLLEGE OF ARTS AND SCIENCES 70 THE SCHOOL OF DENTAL HYGIENE 77 THE COLLEGE OF EDUCATION AND NURSING 79 THE COLLEGE OF TECHNOLOGY 88 THE GRADUATE COLLEGE 97 THE COLLEGE OF MEDICINE 107 THE UNIVERSITY EXTENSION 110 COURSES OF INSTRUCTION 114 THE ALUMNI COUNCIL 195 ENROLLMENT STATISTICS 197 DEGREES AND PRIZES 200 LOAN FUNDS, SCHOLARSHIPS, AND PRIZES 213 GENERAL INDEX '222 ACADEMIC CALENDAR 225 THE UNIVERSITY OF VERMONT J The University is located at Burlington, Vermont, overlooking an at tractive tree-shaded city situated on the shores of Lake Champlain.