A Switch in the Atlantic Oscillation Correlates with Inter-Annual
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

AOU Classification Committee – North and Middle America
AOU Classification Committee – North and Middle America Proposal Set 2016-C No. Page Title 01 02 Change the English name of Alauda arvensis to Eurasian Skylark 02 06 Recognize Lilian’s Meadowlark Sturnella lilianae as a separate species from S. magna 03 20 Change the English name of Euplectes franciscanus to Northern Red Bishop 04 25 Transfer Sandhill Crane Grus canadensis to Antigone 05 29 Add Rufous-necked Wood-Rail Aramides axillaris to the U.S. list 06 31 Revise our higher-level linear sequence as follows: (a) Move Strigiformes to precede Trogoniformes; (b) Move Accipitriformes to precede Strigiformes; (c) Move Gaviiformes to precede Procellariiformes; (d) Move Eurypygiformes and Phaethontiformes to precede Gaviiformes; (e) Reverse the linear sequence of Podicipediformes and Phoenicopteriformes; (f) Move Pterocliformes and Columbiformes to follow Podicipediformes; (g) Move Cuculiformes, Caprimulgiformes, and Apodiformes to follow Columbiformes; and (h) Move Charadriiformes and Gruiformes to precede Eurypygiformes 07 45 Transfer Neocrex to Mustelirallus 08 48 (a) Split Ardenna from Puffinus, and (b) Revise the linear sequence of species of Ardenna 09 51 Separate Cathartiformes from Accipitriformes 10 58 Recognize Colibri cyanotus as a separate species from C. thalassinus 11 61 Change the English name “Brush-Finch” to “Brushfinch” 12 62 Change the English name of Ramphastos ambiguus 13 63 Split Plain Wren Cantorchilus modestus into three species 14 71 Recognize the genus Cercomacroides (Thamnophilidae) 15 74 Split Oceanodroma cheimomnestes and O. socorroensis from Leach’s Storm- Petrel O. leucorhoa 2016-C-1 N&MA Classification Committee p. 453 Change the English name of Alauda arvensis to Eurasian Skylark There are a dizzying number of larks (Alaudidae) worldwide and a first-time visitor to Africa or Mongolia might confront 10 or more species across several genera. -

Classic Versus Metabarcoding Approaches for the Diet Study of a Remote Island Endemic Gecko
Questioning the proverb `more haste, less speed': classic versus metabarcoding approaches for the diet study of a remote island endemic gecko Vanessa Gil1,*, Catarina J. Pinho2,3,*, Carlos A.S. Aguiar1, Carolina Jardim4, Rui Rebelo1 and Raquel Vasconcelos2 1 Centre for Ecology, Evolution and Environmental Changes, Faculdade de Ciências da Universidade de Lisboa, Lisboa, Portugal 2 CIBIO, Centro de Investigacão¸ em Biodiversidade e Recursos Genéticos, InBIO Laboratório Associado, Universidade do Porto, Vairão, Portugal 3 Departamento de Biologia, Faculdade de Ciências da Universidade do Porto, Porto, Portugal 4 Instituto das Florestas e Conservacão¸ da Natureza IP-RAM, Funchal, Madeira, Portugal * These authors contributed equally to this work. ABSTRACT Dietary studies can reveal valuable information on how species exploit their habitats and are of particular importance for insular endemics conservation as these species present higher risk of extinction. Reptiles are often neglected in island systems, principally the ones inhabiting remote areas, therefore little is known on their ecological networks. The Selvagens gecko Tarentola (boettgeri) bischoffi, endemic to the remote and integral reserve of Selvagens Archipelago, is classified as Vulnerable by the Portuguese Red Data Book. Little is known about this gecko's ecology and dietary habits, but it is assumed to be exclusively insectivorous. The diet of the continental Tarentola species was already studied using classical methods. Only two studies have used next-generation sequencing (NGS) techniques for this genus thus far, and very few NGS studies have been employed for reptiles in general. Considering the lack of information on its diet Submitted 10 April 2019 and the conservation interest of the Selvagens gecko, we used morphological and Accepted 22 October 2019 Published 2 January 2020 DNA metabarcoding approaches to characterize its diet. -

Proposals 2018-C
AOS Classification Committee – North and Middle America Proposal Set 2018-C 1 March 2018 No. Page Title 01 02 Adopt (a) a revised linear sequence and (b) a subfamily classification for the Accipitridae 02 10 Split Yellow Warbler (Setophaga petechia) into two species 03 25 Revise the classification and linear sequence of the Tyrannoidea (with amendment) 04 39 Split Cory's Shearwater (Calonectris diomedea) into two species 05 42 Split Puffinus boydi from Audubon’s Shearwater P. lherminieri 06 48 (a) Split extralimital Gracula indica from Hill Myna G. religiosa and (b) move G. religiosa from the main list to Appendix 1 07 51 Split Melozone occipitalis from White-eared Ground-Sparrow M. leucotis 08 61 Split White-collared Seedeater (Sporophila torqueola) into two species (with amendment) 09 72 Lump Taiga Bean-Goose Anser fabalis and Tundra Bean-Goose A. serrirostris 10 78 Recognize Mexican Duck Anas diazi as a species 11 87 Transfer Loxigilla portoricensis and L. violacea to Melopyrrha 12 90 Split Gray Nightjar Caprimulgus indicus into three species, recognizing (a) C. jotaka and (b) C. phalaena 13 93 Split Barn Owl (Tyto alba) into three species 14 99 Split LeConte’s Thrasher (Toxostoma lecontei) into two species 15 105 Revise generic assignments of New World “grassland” sparrows 1 2018-C-1 N&MA Classification Committee pp. 87-105 Adopt (a) a revised linear sequence and (b) a subfamily classification for the Accipitridae Background: Our current linear sequence of the Accipitridae, which places all the kites at the beginning, followed by the harpy and sea eagles, accipiters and harriers, buteonines, and finally the booted eagles, follows the revised Peters classification of the group (Stresemann and Amadon 1979). -
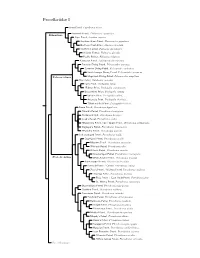
Procellariidae Species Tree
Procellariidae I Snow Petrel, Pagodroma nivea Antarctic Petrel, Thalassoica antarctica Fulmarinae Cape Petrel, Daption capense Southern Giant-Petrel, Macronectes giganteus Northern Giant-Petrel, Macronectes halli Southern Fulmar, Fulmarus glacialoides Atlantic Fulmar, Fulmarus glacialis Pacific Fulmar, Fulmarus rodgersii Kerguelen Petrel, Aphrodroma brevirostris Peruvian Diving-Petrel, Pelecanoides garnotii Common Diving-Petrel, Pelecanoides urinatrix South Georgia Diving-Petrel, Pelecanoides georgicus Pelecanoidinae Magellanic Diving-Petrel, Pelecanoides magellani Blue Petrel, Halobaena caerulea Fairy Prion, Pachyptila turtur ?Fulmar Prion, Pachyptila crassirostris Broad-billed Prion, Pachyptila vittata Salvin’s Prion, Pachyptila salvini Antarctic Prion, Pachyptila desolata ?Slender-billed Prion, Pachyptila belcheri Bonin Petrel, Pterodroma hypoleuca ?Gould’s Petrel, Pterodroma leucoptera ?Collared Petrel, Pterodroma brevipes Cook’s Petrel, Pterodroma cookii ?Masatierra Petrel / De Filippi’s Petrel, Pterodroma defilippiana Stejneger’s Petrel, Pterodroma longirostris ?Pycroft’s Petrel, Pterodroma pycrofti Soft-plumaged Petrel, Pterodroma mollis Gray-faced Petrel, Pterodroma gouldi Magenta Petrel, Pterodroma magentae ?Phoenix Petrel, Pterodroma alba Atlantic Petrel, Pterodroma incerta Great-winged Petrel, Pterodroma macroptera Pterodrominae White-headed Petrel, Pterodroma lessonii Black-capped Petrel, Pterodroma hasitata Bermuda Petrel / Cahow, Pterodroma cahow Zino’s Petrel / Madeira Petrel, Pterodroma madeira Desertas Petrel, Pterodroma -

Shearwatersrefs V1.10.Pdf
Introduction I have endeavoured to keep typos, errors, omissions etc in this list to a minimum, however when you find more I would be grateful if you could mail the details during 2018 & 2019 to: [email protected]. Please note that this and other Reference Lists I have compiled are not exhaustive and are best employed in conjunction with other sources. Grateful thanks to Ashley Fisher (www.scillypelagics.com) for the cover images. All images © the photographer. Joe Hobbs Index The general order of species follows the International Ornithologists' Union World Bird List (Gill, F. & Donsker, D. (eds.) 2017. IOC World Bird List. Available from: http://www.worldbirdnames.org/ [version 7.3 accessed August 2017]). Version Version 1.10 (January 2018). Cover Main image: Great Shearwater. At sea 3’ SW of the Bishop Rock, Isles of Scilly. 8th August 2009. Picture by Ashley Fisher. Vignette: Sooty Shearwater. At sea off the Isles of Scilly. 14th August 2009. Picture by Ashley Fisher. Species Page No. Audubon's Shearwater [Puffinus lherminieri] 34 Balearic Shearwater [Puffinus mauretanicus] 28 Bannerman's Shearwater [Puffinus bannermani] 37 Barolo Shearwater [Puffinus baroli] 38 Black-vented Shearwater [Puffinus opisthomelas] 30 Boyd's Shearwater [Puffinus boydi] 38 Bryan's Shearwater [Puffinus bryani] 29 Buller's Shearwater [Ardenna bulleri] 15 Cape Verde Shearwater [Calonectris edwardsii] 12 Christmas Island Shearwater [Puffinus nativitatis] 23 Cory's Shearwater [Calonectris borealis] 9 Flesh-footed Shearwater [Ardenna carneipes] 21 Fluttering -

A Checklist of Birds of Britain (9Th Edition)
Ibis (2018), 160, 190–240 doi: 10.1111/ibi.12536 The British List: A Checklist of Birds of Britain (9th edition) CHRISTOPHER J. MCINERNY,1,2 ANDREW J. MUSGROVE,1,3 ANDREW STODDART,1 ANDREW H. J. HARROP1 † STEVE P. DUDLEY1,* & THE BRITISH ORNITHOLOGISTS’ UNION RECORDS COMMITTEE (BOURC) 1British Ornithologists’ Union, PO Box 417, Peterborough PE7 3FX, UK 2School of Life Sciences, University of Glasgow, Glasgow G12 8QQ, UK 3British Trust for Ornithology, The Nunnery, Thetford, IP24 2PU, UK Recommended citation: British Ornithologists’ Union (BOU). 2018. The British List: a Checklist of Birds of Britain (9th edition). Ibis 160: 190–240. and the Irish Rare Birds Committee are no longer INTRODUCTION published within BOURC reports. This, the 9th edition of the Checklist of the Birds The British List is under continuous revision by of Britain, referred to throughout as the British BOURC. New species and subspecies are either List, has been prepared as a statement of the status added or removed, following assessment; these are of those species and subspecies known to have updated on the BOU website (https://www.bou. occurred in Britain and its coastal waters (Fig. 1). org.uk/british-list/recent-announcements/) at the time It incorporates all the changes to the British List of the change, but only come into effect on the List up to and including the 48th Report of the British on publication in a BOURC report in Ibis. A list of Ornithologists’ Union Records Committee the species and subspecies removed from the British (BOURC) (BOU 2018), and detailed in BOURC List since the 8th edition is shown in Appendix 1. -

Canary Islands Trip Report Macaronesian Endemics 16Th to 22Nd April 2016
Canary Islands Trip Report Macaronesian Endemics 16th to 22nd April 2016 Houbara Bustard by David Hoddinott Trip Report Compiled by Tour Leader David Hoddinott RBT Canary Islands Trip Report April 216 2 Trip Summary We started our trip in Madrid where we arrived in the late afternoon, settled into a comfortable hotel and then, over a delicious dinner, chatted about the adventures to come on the Canary Islands. The following morning we took a flight to the arid island of Fuerteventura, situated in the eastern Canary Islands group in the Atlantic Ocean, just off Morocco. On arrival, we checked in at our comfortable hotel and then set about enjoying some birding around the nearby Los Molinos Reservoir. Here we notched up a good selection of species including some key targets. Notable sightings included good numbers of Ruddy Shelduck, Common Buzzard, Egyptian Vulture, Little Ringed Plover, Eurasian Hoopoe, Common Kestrel, Northern Raven, Lesser Short-toed Lark, Spectacled Warbler and Trumpeter Finch. Both Canary Islands Stonechat, which is endemic to Fuerteventura, and Berthelot’s Pipit, a Macaronesian endemic, gave Trumpeter Finch by David Hoddinott splendid views and so we returned to the hotel chuffed with our afternoon’s birding and excited in anticipation of the birding the following day. The following morning we departed early for the drive to the area around La Oliva in search of the most sought after bird of the trip. What else but the great Houbara Bustard! It’s not endemic to the islands, however the Canary Islands are certainly the best place in the world to see this impressive bird. -

Second Record of Bryan's Shearwater Puffinus
Pyle et al.: Bryan’s Shearwater in Midway Atoll 5 SECOND RECORD OF BRYAN’S SHEARWATER PUFFINUS BRYANI FROM MIDWAY ATOLL, WITH NOTES ON HABITAT SELECTION, VOCALIZATIONS AND AT-SEA DISTRIBUTION PETER PYLE1, REGINALD DAVID2, BRUCE D. EILERTS3, A. BINION AMERSON4, ABRAHAM BORKER5 & MATTHEW MCKOWN5 1The Institute for Bird Populations, PO Box 1346, Point Reyes Station, CA 94956, USA ([email protected]) 2Rana Productions, 75-5815 Kini Loop, Kailua-Kona, HI 96740, USA 3The Sandberg Group, Inc., 14565 Valley View Ave., Santa Fe Springs, CA 90670, USA 413723 Littlecrest Drive, Dallas, TX 75234, USA 5Coastal Conservation Action Lab, University of California Santa Cruz, 100 Shaffer Road, Santa Cruz, CA 95060, USA Submitted 27 September 2011, accepted 8 October 2013 SUMMARY PYLE, P., DAVID, R., EILERTS, B.D., AMERSON, A.B., BORKER, A. & McKOWN, M. 2014. Second record of Bryan’s Shearwater Puffinus bryani from Midway Atoll, with notes on habitat selection, vocalizations and at-sea distribution. Marine Ornithology 42: 5–8. Little is known about the conservation requirements of Bryan’s Shearwater Puffinus bryani, first described in 2011 based on a specimen collected in February 1963 near an area containing concrete rubble at Midway Atoll. Here we document a second Bryan’s Shearwater observed on Midway during the winters of 1990/91 and 1991/92. It was vocalizing from a 0.5–0.7 m crevice within an accumulation of artificial concrete and coral rubble. Recent winter specimens of Bryan’s Shearwaters from the Bonin (Ogasawara) Islands, Japan, were also collected in areas with rocky crevices, possibly burrows they co-utilize with summer-breeding Bulwer’s Petrels Bulweria bulwerii. -

The Scientific Role of Malcolm Clarke in the Azores
The scientific role of Malcolm Clarke in the Azores Abstract Malcolm R. Clarke (1930 – 2013) was a British teuthologist who made an important contribution to marine science in the Azores archipelago (Portugal). An authority in the study of oceanic cephalopods during the second half of the XXth century ‐ particularly in the use of stomach contents to understand both prey and predators ‐ he started doing research in the Azores from 1980’s onward, settling for residency after his retirement in 2000. He is the lead or contributing author of ca. 30% high scientific impact publications resulting from a search for “Azores” and “cephalopod” (source Science Direct, June‐2013), spanning from 1981 to 2012. Malcolm believed the way to understand the marine ecosystem was through sampling commercially exploited and top predator species, thus allowing to i) identify how species interrelate, ii) understand which species/organisms are critical to sustain the present balance and iii) determine how the island and the oceanic ecosystem interact. His research in the Azores focused on two main of areas: dietary studies from cephalopod predators and the ecology of cephalopods on seamounts. Since his first visit in 1981, he was involved in the description of the dietary ecology of several cetaceans, large pelagic fishes, deep‐water fishes and seabirds. Regarding seamount ecology, Malcolm revised the association of cephalopods with seamounts, by using his own data, updating and enlarging the different cephalopod groups according to species behavior and ecology. Malcolm taught several students working in the Azores on cephalopods and beak identification, lecturing the Third International Workshop in Faial (2007). -

Driven by the Moon: the Foraging Behaviour of Procellariiforms at Night
Eduardo Castro Lobato Driven by the moon: the foraging behaviour of Procellariiforms at night Dissertação apresentada à Universidade de Coimbra para cumprimento dos requisitos necessários à obtenção do grau de Mestre em Ecologia, realizada sob a orientação científica do Doutor Vítor Hugo Rodrigues Paiva (Universidade de Coimbra) e do Professor Doutor Jaime Albino Ramos (Universidade de Coimbra) Agosto/2017 Driven by the moon: the foraging behaviour of Procellariiforms at night Dissertação apresentada à Universidade de Coimbra para cumprimento dos requisitos necessários à obtenção do grau de Mestre em Ecologia, realizada sob a orientação científica do Doutor Vítor Hugo Rodrigues Paiva (Universidade de Coimbra) e do Professor Doutor Jaime Albino Ramos (Universidade de Coimbra). Eduardo Castro Lobato Departamento de Ciências da Vida Universidade de Coimbra Coimbra|2017 ACKNOWLEDGEMENTS After completing this master thesis, I must thank all of those who in some way contributed to its realization: First, for my supervisors Dr. Vitor Paiva, Prof. Dr. Jaime Ramos, for helping me on this process, for all guidance, patience, reviews and friendship. A special thanks to Dr. Vitor Paiva for the relentless availability, perseverance and confidence deposited in me, for the sharing of knowledge and advice given and for making me believe it was possible to complete this thesis. Also thank both of you for presenting me with this theme, without your support this work would not be possible. Thank you for be such nice persons and dedicated to me. Also a thanks for team in the Lab, that receive me with open arms and help me preparing the presentation of the seminars, thanks for the tips and advices given. -

Spatio-Temporal Variation in the Diet of Cory's Shearwater Calonectris
Deep-Sea Research I 70 (2012) 1–13 Contents lists available at SciVerse ScienceDirect Deep-Sea Research I journal homepage: www.elsevier.com/locate/dsri Spatio-temporal variation in the diet of Cory’s shearwater Calonectris diomedea in the Azores archipelago, northeast Atlantic Vero´ nica Neves a,n,1, Dirk Nolf b, Malcolm Clarke c a Departamento de Oceanografia e Pescas, Centro do IMAR da Universidade dos Ac-ores, Rua Prof. Dr. Frederico Machado no. 4, 9901-862 Horta, Faial, Azores, Portugal b Institut Royal des Sciences Naturelles de Belgique (IRSNB), 29 rue Vautier, B-1000 Bruxelles, Belgique c Rua do Porto 18, 9930-430 Sao~ Joao,~ Pico, Azores, Portugal article info abstract Article history: The diet of Cory’s shearwater Calonectris diomedea in the Azores was studied in four islands of the Received 17 May 2012 archipelago over four breeding seasons, using stomach flushings from 959 birds. Fish were identified Received in revised form from flesh, otoliths and vertebrae and cephalopods from flesh and lower beaks. The frequency of 14 August 2012 occurrence of prey taxa, and the numerical frequency of fish and cephalopods, showed marked Accepted 22 August 2012 variations both spatially, across the breeding cycle and between years. Overall, cephalopods from 37 Available online 31 August 2012 species representing 17 families and fish from 33 species representing 18 families were identified, Keywords: representing over 70 prey species and trebling what was previously known. Histioteuthidae, Cranchiidae Ommastrephidae and Cranchiidae were the only cephalopod families present every year and Blue jack mackerel represented two thirds of the cephalopods’ consumption by number. -

AOU Check-List Supplement
AOU Check-list Supplement The Auk 117(3):847–858, 2000 FORTY-SECOND SUPPLEMENT TO THE AMERICAN ORNITHOLOGISTS’ UNION CHECK-LIST OF NORTH AMERICAN BIRDS This first Supplement since publication of the 7th Icterus prosthemelas, Lonchura cantans, and L. atricap- edition (1998) of the AOU Check-list of North American illa); (3) four species are changed (Caracara cheriway, Birds summarizes changes made by the Committee Glaucidium costaricanum, Myrmotherula pacifica, Pica on Classification and Nomenclature between its re- hudsonia) and one added (Caracara lutosa) by splits constitution in late 1998 and 31 January 2000. Be- from now-extralimital forms; (4) four scientific cause the makeup of the Committee has changed sig- names of species are changed because of generic re- nificantly since publication of the 7th edition, it allocation (Ibycter americanus, Stercorarius skua, S. seems appropriate to outline the way in which the maccormicki, Molothrus oryzivorus); (5) one specific current Committee operates. The philosophy of the name is changed for nomenclatural reasons (Baeolo- Committee is to retain the present taxonomic or dis- phus ridgwayi); (6) the spelling of five species names tributional status unless substantial and convincing is changed to make them gramatically correct rela- evidence is published that a change should be made. tive to the generic name (Jacamerops aureus, Poecile The Committee maintains an extensive agenda of atricapilla, P. hudsonica, P. cincta, Buarremon brunnein- potential action items, including possible taxonomic ucha); (7) one English name is changed to conform to changes and changes to the list of species included worldwide use (Long-tailed Duck), one is changed in the main text or the Appendix.