Dormancy and Germination of Annona Macroprophyllata (Annonaceae): the Importance of the Micropylar Plug and Seed Position in the Fruits
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Guide to Foreign Language Programs, North Carolina
DOCUMENT RESUME ED 419 387 FL 025 196 TITLE Cover to Cover: A Guide to Foreign Language Programs, Instruction and Resources. INSTITUTION North Carolina State Dept. of Public Instruction, Raleigh. Instructional Services. PUB DATE 1997-00-00 NOTE 310p. PUB TYPE Guides Classroom Teacher (052) EDRS PRICE MF01/PC13 Plus Postage. DESCRIPTORS Classroom Techniques; Course Evaluation; *Curriculum Design; Curriculum Development; Disabilities; Distance Education; Educational Research; Elementary Secondary Education; English (Second Language); Graduation Requirements; Higher Education; Instructional Effectiveness; Instructional Materials; International Educational Exchange; Media Selection; Parent Participation; Professional Associations; School Business Relationship; School Community Relationship; Second Language Instruction; *Second Language Programs; *State Standards; *Student Evaluation; Student Motivation; Study Abroad; Teaching Methods; Teaching Skills; Travel IDENTIFIERS North Carolina ABSTRACT The guide is intended as a resource for North Carolina teachers and administrators concerning second language education. Part 1 offers a rationale for second language education, and provides specific reasons for studying French, German, Latin, Spanish, and uncommonly taught languages. Part 2 describes instructional program models for elementary and middle schools, outlines st; e high school graduation and academic degree requirements, describes advaled placement courses, outlines the international baccalaureate :ogram, and provides data on language -

Within-Tree Distribution of Seven Inseet Pests of Soursop (Annona Muricata) in Hondurasl
Within-tree distribution of seven inseet pests of soursop (Annona muricata) in Hondurasl Carlos A. Granadino and Ronald D. Cave2 Abstract. Thc wilh¡n·trce distributions of scvell insect pesls of s()ursop, AI/Ilona 111 11 rÚ'll la L., were dctcrIllincd during a l-ycar period at four localilics in Honduras. Five foliage and slem reeders, Corythucha gossypii (E), Clllloconophora caliginosa (Walker), Membracis mexicana (Guerin), Parasaissetia nigra (NiCtncr) and Saissetia o/eae Olivier, and two fruit/secd borers, Bephratelloides clIbensis (Ashmead) and Cerconota anonella ¡Sepp), were studied. [nfcstations by C. goss.l'pii, C. ca/iginosa, M. mexicana and the fruiliseed borers were grealesl in the middlc third of the tree canopy. Dcnsities of P. nigra and S. o/eae were highest in lhe middle and bottom seetions. Infeslations by P. nigm were greater on the southern hall' of trees, whereas inkstations by C. anonella were greater on the northern half. Key words: Spatial distribution. foliage pests, slem pests, fruit borers, seed borers, sampling. Resumen: Se determinó la distribución de siete plagas insccLilcs en la copa de árboles de guanábana, An/l(ma JIIurica'" L.. duran le un año en cuatro localidades en Honduras. Se estudiaron cinco especies, Coryt/1I/cha goss)pii (F.), C"I!oCot/op/lOra caliginoJo (Walker), Membracis mexicano (Guerin). Parasaissetia nigra (Nietner) y SaisselÍa o/eae Olivier, que atacan el follaje y los lallos. y dos especies, Bephratelloides cubemis (Ashmead) y Cerconota anollella (Sepp), que son barrenadores del fruto y semilla. Las infestaciones por C. go.\Sypii, C. ca/iginosa. M. mexicana y los barrenadores fueron mayores en el tcrcio medio de la copa del árbol. -

ISTA List of Stabilised Plant Names 7Th Edition
ISTA List of Stabilised Plant Names 7th Edition ISTA Nomenclature Committee Chair Dr. M. Schori Published by All rights reserved. No part of this publication may be The International Seed Testing Association (ISTA) reproduced, stored in any retrieval system or transmitted in Richtiarkade 18, CH- 8304 Wallisellen, Switzerland any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without prior ©2021 International Seed Testing Association (ISTA) permission in writing from ISTA. ISBN 978-3-906549-77-4 Valid from: 16.06.2021 ISTA List of Stabilised Plant Names 1st Edition 1966 ISTA Nomenclature Committee Chair: Prof P. A. Linehan 2nd Edition 1983 ISTA Nomenclature Committee Chair: Dr. H. Pirson 3rd Edition 1988 ISTA Nomenclature Committee Chair: Dr. W. A. Brandenburg 4th Edition 2001 ISTA Nomenclature Committee Chair: Dr. J. H. Wiersema 5th Edition 2007 ISTA Nomenclature Committee Chair: Dr. J. H. Wiersema 6th Edition 2013 ISTA Nomenclature Committee Chair: Dr. J. H. Wiersema 7th Edition 2019 ISTA Nomenclature Committee Chair: Dr. M. Schori 7th Edition 2 ISTA List of Stabilised Plant Names Table of Contents A .............................................................................................................................................................. 7 B ............................................................................................................................................................ 21 C ........................................................................................................................................................... -

Appliances Appliances
R'-J --vfl ff ■'vefl - -I m ■ 'I / WEDNESDAY, SEPTEMBER tS, 1960 'TAGE TWEKTY-EiGHT dlanirl|i«atifr O w nin g AT«ng9 DaQjr Net Pres* Ron Thff WMtiMr Fer thS W M k Kndoi ratoeaat a t V . B HtellMF MfiiffB i < J S^rtOAitOM Chmtg, nOs as fffitolp 1 A spaghetti supper and social Lawren'ce A. Herzog, 82 Vernon prepared by the National CotincU Classes to Start of Churches, entitled "Literacy Un Gibbons Assembly algOit. tm r -mass- 99. - sponsored by the walther League 8L, a senior at the University of 13,220 elougy tineemw wtto aai About Town of Zion Evangelical Lutheran Connecticut, has received a cita locking .the Bible." He described ehhwm. BOgh near 79. Church will be held Saturday at tion naming him a "distinguished In Boat Handling the efforts of Christian missions to Plans Card Party NT at Mm Aadtt The board of directors of Man 6:80 p.m. at the church. For reser military student” at the univer teach Bible concepts while teach loC' M anche8t€r~^A C ity o f VU lagi'Cfutrm chester Girl Scouts, Inc., will meet vations, those Interested may call sity. He is an Army ROTC cadet., • ’The Hartford Power ’ Squadron ing illiterates to read. Such a pro Gibbons Assembly, Catholic gram, he said, strengthens both ' - •4'^' . / at the Gin Scout olBce next TUes- Howard Hansen, 21 Bremen Rd., will agsdn conduct classes in pi Ladles of Columbus, will sponsors: ''.""" ---------------r - -------------------- ---------------- ■ ' " " V ' *■* day at 7:30 p.tn. reading and evangelism. What U military whist and setback party K f f C B I I K O (OIXMlIlad AdvarlMag am Toga N ) by tomorrow evening. -

Forest Inventory and Analysis National Core Field Guide
National Core Field Guide, Version 5.1 October, 2011 FOREST INVENTORY AND ANALYSIS NATIONAL CORE FIELD GUIDE VOLUME I: FIELD DATA COLLECTION PROCEDURES FOR PHASE 2 PLOTS Version 5.1 National Core Field Guide, Version 5.1 October, 2011 Changes from the Phase 2 Field Guide version 5.0 to version 5.1 Changes documented in change proposals are indicated in bold type. The corresponding proposal name can be seen using the comments feature in the electronic file. • Section 8. Phase 2 (P2) Vegetation Profile (Core Optional). Corrected several figure numbers and figure references in the text. • 8.2. General definitions. NRCS PLANTS database. Changed text from: “USDA, NRCS. 2000. The PLANTS Database (http://plants.usda.gov, 1 January 2000). National Plant Data Center, Baton Rouge, LA 70874-4490 USA. FIA currently uses a stable codeset downloaded in January of 2000.” To: “USDA, NRCS. 2010. The PLANTS Database (http://plants.usda.gov, 1 January 2010). National Plant Data Center, Baton Rouge, LA 70874-4490 USA. FIA currently uses a stable codeset downloaded in January of 2010”. • 8.6.2. SPECIES CODE. Changed the text in the first paragraph from: “Record a code for each sampled vascular plant species found rooted in or overhanging the sampled condition of the subplot at any height. Species codes must be the standardized codes in the Natural Resource Conservation Service (NRCS) PLANTS database (currently January 2000 version). Identification to species only is expected. However, if subspecies information is known, enter the appropriate NRCS code. For graminoids, genus and unknown codes are acceptable, but do not lump species of the same genera or unknown code. -

Nomenclatural and Taxonomic Notes on Annona (Annonaceae)
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Annalen des Naturhistorischen Museums in Wien Jahr/Year: 2001 Band/Volume: 103B Autor(en)/Author(s): Rainer H. Artikel/Article: Nomenclatural and taxonomic notes on Annona (Annonaceae). 513-524 ©Naturhistorisches Museum Wien, download unter www.biologiezentrum.at Ann. Naturhist. Mus. Wien 103 B 513-524 Wien, Dezember 2001 Nomenclatural and taxonomic notes on Annona (Annonaceae) H. Rainer* Abstract In the course of monographic studies on the genus Annona (Annonaceae) some cases of nomenclatural and taxonomic changes and need for typifications turned up and are herewith presented. Raimondia is included in Annona due to its general resemblance in morphological and anatomical characters, its species already described as Annona are reestablished, and one new combination is made. The new combinations are Annona cacans WARM, subsp. glabriuscula (R.E.FR.) H.RAWER and Annona deceptrix (WESTRA) H.RAINER. Key words: Annonaceae, Annona, Raimondia, Flora Neotropica, typification. Zusammenfassung Während der monographischen Studien an der Gattung Annona (Annonaceae) wurden einige nomenkla- torische und taxonomische Änderungen evident, sowie in einigen Fällen Typifizierungen notwendig, die hier präsentiert werden. Die Gattung Raimondia wird wegen ihrer weitgehenden Übereinstimmung in mor- phologischen wie anatomischen Merkmalen in Annona eingegliedert. Die schon unter Annona beschriebe- nen Arten werden wiederhergestellt und eine Neukombination durchgeführt. Die beiden Neukombinationen betreffen Annona cacans WARM, subsp. glabriuscula (R.E.FR.) H.RAINER und Annona deceptrix (WESTRA) H.RAINER. Introduction In the course of the studies for a monograph of the neotropical taxa of the genus Annona (Annonaceae), the number of collections increased substantially compared to the mate- rial available to FRIES (1931), the last comprehensive treatment of the genus. -

With Annona Macroprophyllata Fruits in Orchards of Chiapas, Mexico
ISSN Printed: 0034-7744 ISSN digital: 2215-2075 DOI 10.15517/rbt.v69i1.43827 Interaction of Bephratelloides cubensis (Hymenoptera: Eurytomidae) with Annona macroprophyllata fruits in orchards of Chiapas, Mexico José Norman González-Sánchez1, Alma Rosa González-Esquinca1, Claudia Azucena Durán-Ruiz1, Iván De-la-Cruz-Chacón1 & Marisol Castro-Moreno1* 1. Universidad de Ciencias y Artes de Chiapas, Laboratorio de Fisiología y Química Vegetal, Instituto de Ciencias Biológicas, Tuxtla Gutiérrez, Chiapas, 29000, Mexico; [email protected], [email protected], [email protected], [email protected], [email protected] (*Correspondencia). Received 11-IX-2020. Corrected 09-XII-2020. Accepted 17-XII-2020. ABSTRACT. Introduction: Annona macroprophyllata Donn. Smith. (Annonaceae) (syn. Annona diversifolia Saff.) is a valued fruit tree species known as papausa. In Mexico and Central America, this fruit has become an important crop because of its tasty flavor and high pulp content. Its fruits are frequently damaged by the inci- dence of wasps of the genus Bephratelloides Girault (Hymenoptera: Eurytomidae), which develop inside the seeds. Objective: to report the interaction of Bephratelloides cubensis Ashmead during its life cycle in fruits of A. macroprophyllata. Methods: We periodically collected fruits in different states of growth recording a) oviposition, b) the moment of evident infection, c) the development of the wasps inside the seeds, and d) their emergence as adults. We also determined the proportion of damaged fruits and seeds. Results: The data indi- cate that wasps preferred to oviposit on fruits with a diameter of less than 8 cm, oviposition was more frequent between 11:00 am and 03:00 pm., and there was 26 % infestation of fruits, and 9 % of seeds. -

The One Hundred Tree Species Prioritized for Planting in the Tropics and Subtropics As Indicated by Database Mining
The one hundred tree species prioritized for planting in the tropics and subtropics as indicated by database mining Roeland Kindt, Ian K Dawson, Jens-Peter B Lillesø, Alice Muchugi, Fabio Pedercini, James M Roshetko, Meine van Noordwijk, Lars Graudal, Ramni Jamnadass The one hundred tree species prioritized for planting in the tropics and subtropics as indicated by database mining Roeland Kindt, Ian K Dawson, Jens-Peter B Lillesø, Alice Muchugi, Fabio Pedercini, James M Roshetko, Meine van Noordwijk, Lars Graudal, Ramni Jamnadass LIMITED CIRCULATION Correct citation: Kindt R, Dawson IK, Lillesø J-PB, Muchugi A, Pedercini F, Roshetko JM, van Noordwijk M, Graudal L, Jamnadass R. 2021. The one hundred tree species prioritized for planting in the tropics and subtropics as indicated by database mining. Working Paper No. 312. World Agroforestry, Nairobi, Kenya. DOI http://dx.doi.org/10.5716/WP21001.PDF The titles of the Working Paper Series are intended to disseminate provisional results of agroforestry research and practices and to stimulate feedback from the scientific community. Other World Agroforestry publication series include Technical Manuals, Occasional Papers and the Trees for Change Series. Published by World Agroforestry (ICRAF) PO Box 30677, GPO 00100 Nairobi, Kenya Tel: +254(0)20 7224000, via USA +1 650 833 6645 Fax: +254(0)20 7224001, via USA +1 650 833 6646 Email: [email protected] Website: www.worldagroforestry.org © World Agroforestry 2021 Working Paper No. 312 The views expressed in this publication are those of the authors and not necessarily those of World Agroforestry. Articles appearing in this publication series may be quoted or reproduced without charge, provided the source is acknowledged. -

Annona Diversifolia) EN MADUREZ %, Had the Highest Values in Material Handled in MA
Nota Científica Rev. Fitotec. Mex. Vol. 35 (Núm. Especial 5): 75 - 81, 2012 gars, whose average values were 0.20 %, 17.9 ºBrix and 17.6 %, respecti- COMPORTAMIENTO POSTCOSECHA DE FRUTOS vely. However, reducing sugars content, which varied between 4 and 10 DE ILAMA (Annona diversifolia) EN MADUREZ %, had the highest values in material handled in MA. The modified at- COMESTIBLE ALMACENADOS EN ATMÓSFERA mosphere proved to be useful for reducing weight losses and softening MODIFICADA rate of ilama fruits, thus allowing longer postharvest storage periods. Index words: Annona diversifolia, papausa, modified atmospheres, postharvest quality. INTRODUCCIÓN POSTHARVEST BEHAVIOR OF ILAMA (Annona diversifolia) FRUITS AT EDIBLE MATURITY La familia de las Anonáceas comprende alrededor de 50 STORED IN A MODIFIED ATMOSPHERE géneros, entre los cuales Annona, Rollinia y Asimina pro- ducen frutos comestibles y los dos primeros son de impor- tancia comercial (George y Nissen, 2003). La mayoría de las especies del género Annona provienen de Suramérica y Salvador Valle-Guadarrama1*, Xóchitl G. América Central (Pinto, 2005); México se reconoce como Ruiz-Sánchez1, Crescenciano Saucedo-Veloz2, Adalberto centro de origen de Annona longifolia (Jalisco) y A. longipes Gómez-Cruz1 y Lila M. Marroquín-Andrade3 (Veracruz), en tanto que la región del Sureste de México y Guatemala es el origen de A. diversifolia, A. purpurea y A. scleroderma (Ferreira y Pinto, 2005). 1Departamento de Ingeniería Agroindustrial y 3Departamento de Fitotec- nia, Universidad Autónoma Chapingo (UACh). Carr. México-Texcoco Las anonas son consumidas como frutos frescos, pro- km 38.5, 56230, Chapingo, Edo. de México, México. 2Fruticultura, Co- legio de Postgraduados, Carr. México-Texcoco km 36.5, 56230, México, ductos semi-procesados y procesados, especialmente en México. -

Review of Investigations on the Annona Species
NOONAN: ANNONA INVESTIGATIONS 205 north-west exposure. The leaders tend to with sulphur powder, NKP, minor elements crawl over the citrus in a south-westerly direc with increased magnesium applications and tion which was also the general orientation of heavy mulching is indicated. the main body of foliage of the mature vines Ten. Scattered flowering throughout the on the Montgomery estate. year was observed on cuttings. Conclusions Eleven. In general, the results of these ori- entative investigations are disappointing from Although further investigations must be a time element viewpoint. For commercial made, the results to date do not indicate that utilization self-compatible naturally-fruiting Strophanthus sarmentosus in South Florida high-yielding strains will have to be bred, will be developed into an economically work which requires a long term breeding selection able crop plant in the near future. The response to hand pollination was suc program. LITERATURE CITED cessful from a breeding standpoint but not 1. Azoff, M. B. and Irvine, J. E. 1952. Paper pre from a commercial standpoint. Breeding and sented at the 17th Annual Meeting of the Florida Academy of Science, Gainesville, Fla., Dec. 13. selection of self-fertile, high-yielding plants is 2. Callon, R. K., Meickle, R. D., and Taylor, W. I. the only rational solution to the problem; 1951. The Source of Sarmentogenin, Chemistry and Industry, No. 17, April 28, pp. 336-7. then clone plantings will be the first commer 3. Creech, J. L., and Dowdle, R. F. 1952. Propa gation of Strophanthus, Economic Botany, Vol. 6, No. cial step. Such a program would require large 1, Jan.-Mar., pp. -

Perennial Edible Fruits of the Tropics: an and Taxonomists Throughout the World Who Have Left Inventory
United States Department of Agriculture Perennial Edible Fruits Agricultural Research Service of the Tropics Agriculture Handbook No. 642 An Inventory t Abstract Acknowledgments Martin, Franklin W., Carl W. Cannpbell, Ruth M. Puberté. We owe first thanks to the botanists, horticulturists 1987 Perennial Edible Fruits of the Tropics: An and taxonomists throughout the world who have left Inventory. U.S. Department of Agriculture, written records of the fruits they encountered. Agriculture Handbook No. 642, 252 p., illus. Second, we thank Richard A. Hamilton, who read and The edible fruits of the Tropics are nnany in number, criticized the major part of the manuscript. His help varied in form, and irregular in distribution. They can be was invaluable. categorized as major or minor. Only about 300 Tropical fruits can be considered great. These are outstanding We also thank the many individuals who read, criti- in one or more of the following: Size, beauty, flavor, and cized, or contributed to various parts of the book. In nutritional value. In contrast are the more than 3,000 alphabetical order, they are Susan Abraham (Indian fruits that can be considered minor, limited severely by fruits), Herbert Barrett (citrus fruits), Jose Calzada one or more defects, such as very small size, poor taste Benza (fruits of Peru), Clarkson (South African fruits), or appeal, limited adaptability, or limited distribution. William 0. Cooper (citrus fruits), Derek Cormack The major fruits are not all well known. Some excellent (arrangements for review in Africa), Milton de Albu- fruits which rival the commercialized greatest are still querque (Brazilian fruits), Enriquito D. -
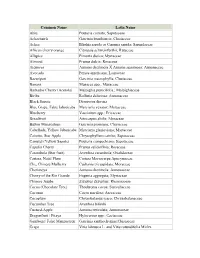
Common Name Latin Name Abiu
Common Name Latin Name Abiu Pouteria caimito; Sapotaceae Achachairú Garcinia brasiliensis; Clusiaceae Ackee Blighia sapida or Cupania sapida; Sapindaceae African cherry orange Citropsis schweinfurthii; Rutaceae Allspice Pimenta dioica; Myrtaceae Almond Prunus dulcis; Rosaceae Atemoya Annona cherimola X Annona squamosa; Annonaceae Avocado Persea americana; Lauraceae Bacuripari Garcinia macrophylla; Clusiaceae Banana Musacea spp.; Musaceae Barbados Cherry (Acerola) Malpighia punicifolia.; Malpighiaceae Biriba Rollinia deliciosa; Annonaceae Black Sapote Diospyros digyna Blue Grape, False Jaboticaba Myrciaria vexator; Myrtaceae Blueberry Vaccinium spp.; Ericaceae Breadfruit Artocarpus altilis; Moraceae Button Mangosteen Garcinia prainiana; Clusiaceae Cabelluda, Yellow Jaboticaba Myrciaria glazioviana; Myrtaceae Caimito, Star Apple Chrysophyllum cainito; Sapotaceae Canistel (Yellow Sapote) Pouteria campechiana; Sapotaceae Capulin Cherry Prunus saliciofloia; Rosaceae Carambola (Star fruit) Averrhoa carambola; Oxalidaceae Carissa, Natal Plum Carissa Macrocarpa;Apocynaceae Che, Chinese Mulberry Cudrania tricuspidata; Moraceae Cherimoya Annona cherimola; Annonaceae Cherry of the Rio Grande Eugenia aggregata; Myrtaceae Chinese Jujube Ziziphus Zizyphus; Rhamnaceae Cocoa (Chocolate Tree) Theobroma cocoa; Sterculiaceae Coconut Cocos nucifera; Arecaceae Cocoplum Chrysobalanus icaco; Chrysobalanaceae Cucumber Tree Averrhoa bilimbi Custard-Apple Annona reticulata; Annonaceae Dragonfruit / Pitaya Hylocereus spp.; Cactaceae Gamboge/ False Mangosteen Garcinia