7: References
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nitric Oxide in Health and Disease of the Nervous System H-Y Yun1,2, VL Dawson1,3,4 and TM Dawson1,3
Molecular Psychiatry (1997) 2, 300–310 1997 Stockton Press All rights reserved 1359–4184/97 $12.00 PROGRESS Nitric oxide in health and disease of the nervous system H-Y Yun1,2, VL Dawson1,3,4 and TM Dawson1,3 Departments of 1Neurology; 3Neuroscience; 4Physiology, Johns Hopkins University School of Medicine, Baltimore, MD, USA Nitric oxide (NO) is a widespread and multifunctional biological messenger molecule. It mediates vasodilation of blood vessels, host defence against infectious agents and tumors, and neurotransmission of the central and peripheral nervous systems. In the nervous system, NO is generated by three nitric oxide synthase (NOS) isoforms (neuronal, endothelial and immunologic NOS). Endothelial NOS and neuronal NOS are constitutively expressed and acti- vated by elevated intracellular calcium, whereas immunologic NOS is inducible with new RNA and protein synthesis upon immune stimulation. Neuronal NOS can be transcriptionally induced under conditions such as neuronal development and injury. NO may play a role not only in physiologic neuronal functions such as neurotransmitter release, neural development, regeneration, synaptic plasticity and regulation of gene expression but also in a variety of neurological disorders in which excessive production of NO leads to neural injury. Keywords: nitric oxide synthase; endothelium-derived relaxing factor; neurotransmission; neurotoxic- ity; neurological diseases Nitric oxide is probably the smallest and most versatile NO synthases isoforms and regulation of NO bioactive molecule identified. Convergence of multi- generation disciplinary efforts in the field of immunology, cardio- vascular pharmacology, chemistry, toxicology and neu- NO is formed by the enzymatic conversion of the guan- robiology led to the revolutionary novel concept of NO idino nitrogen of l-arginine by NO synthase (NOS). -

Dichloroethylene
Development Support Document Final, October 15, 2007 Accessible 2013 1,1-Dichloroethylene CAS Registry Number: 75-35-4 Prepared by Shannon Ethridge, M.S. Toxicology Section Chief Engineer’s Office ___________________________________________________________ TEXAS COMMISSION ON ENVIRONMENTAL QUALITY 1,1 Dichloroethylene Page 2 TABLE OF CONTENTS CHAPTER 1 SUMMARY TABLES ........................................................................................... 4 CHAPTER 2 MAJOR USES OR SOURCES ............................................................................ 6 CHAPTER 3 ACUTE EVALUATION ....................................................................................... 6 3.1 HEALTH-BASED ACUTE REV AND ESL ................................................................................. 6 3.1.1 Physical/Chemical Properties and Key Studies ............................................................. 6 3.1.2 Mode-of-Action (MOA) Analysis ................................................................................. 10 3.1.3 Dose Metric .................................................................................................................. 10 3.1.4 Point of Departure (POD) for the Key Study............................................................... 10 3.1.5 Dosimetric Adjustments ............................................................................................... 11 3.1.6 Critical Effect and Adjustment of PODHEC .................................................................. 11 3.1.7 Health-Based -

Health Effects Support Document for Hexachlorobutadiene Health Effects Support Document for Hexachlorobutadiene
Health Effects Support Document for Hexachlorobutadiene Health Effects Support Document for Hexachlorobutadiene U.S. Environmental Protection Agency Office of Water (4304T) Health and Ecological Criteria Division Washington, DC 20460 www.epa.gov/safewater/ EPA 822-R-03-002 February 2003 Printed on Recycled Paper FOREWORD The Safe Drinking Water Act (SDWA), as amended in 1996, requires the Administrator of the Environmental Protection Agency (EPA) to establish a list of contaminants to aid the agency in regulatory priority setting for the drinking water program. In addition, SDWA requires EPA to make regulatory determinations for no fewer than five contaminants by August 2001. The criteria used to determine whether or not to regulate a chemical on the CCL are the following: The contaminant may have an adverse effect on the health of persons. The contaminant is known to occur or there is a substantial likelihood that the contaminant will occur in public water systems with a frequency and at levels of public health concern. In the sole judgment of the administrator, regulation of such contaminant presents a meaningful opportunity for health risk reduction for persons served by public water systems. The Agency’s findings for the three criteria are used in making a determination to regulate a contaminant. The Agency may determine that there is no need for regulation when a contaminant fails to meet one of the criteria. This document provides the health effects basis for the regulatory determination for hexachlorobutadiene. In arriving at the regulatory determination, data on toxicokinetics, human exposure, acute and chronic toxicity to animals and humans, epidemiology, and mechanisms of toxicity were evaluated. -
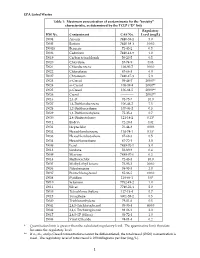
EPA Listed Wastes Table 1: Maximum Concentration of Contaminants For
EPA Listed Wastes Table 1: Maximum concentration of contaminants for the toxicity characteristic, as determined by the TCLP (D list) Regulatory HW No. Contaminant CAS No. Level (mg/L) D004 Arsenic 7440-38-2 5.0 D005 Barium 7440-39-3 100.0 D0018 Benzene 71-43-2 0.5 D006 Cadmium 7440-43-9 1.0 D019 Carbon tetrachloride 56-23-5 0.5 D020 Chlordane 57-74-9 0.03 D021 Chlorobenzene 108-90-7 100.0 D022 Chloroform 67-66-3 6.0 D007 Chromium 7440-47-3 5.0 D023 o-Cresol 95-48-7 200.0** D024 m-Cresol 108-39-4 200.0** D025 p-Cresol 106-44-5 200.0** D026 Cresol ------------ 200.0** D016 2,4-D 94-75-7 10.0 D027 1,4-Dichlorobenzene 106-46-7 7.5 D028 1,2-Dichloroethane 107-06-2 0.5 D029 1,1-Dichloroethylene 75-35-4 0.7 D030 2,4-Dinitrotoluene 121-14-2 0.13* D012 Endrin 72-20-8 0.02 D031 Heptachlor 76-44-8 0.008 D032 Hexachlorobenzene 118-74-1 0.13* D033 Hexachlorobutadiene 87-68-3 0.5 D034 Hexachloroethane 67-72-1 3.0 D008 Lead 7439-92-1 5.0 D013 Lindane 58-89-9 0.4 D009 Mercury 7439-97-6 0.2 D014 Methoxychlor 72-43-5 10.0 D035 Methyl ethyl ketone 78-93-3 200.0 D036 Nitrobenzene 98-95-3 2.0 D037 Pentachlorophenol 87-86-5 100.0 D038 Pyridine 110-86-1 5.0* D010 Selenium 7782-49-2 1.0 D011 Silver 7740-22-4 5.0 D039 Tetrachloroethylene 127-18-4 0.7 D015 Toxaphene 8001-35-2 0.5 D040 Trichloroethylene 79-01-6 0.5 D041 2,4,5-Trichlorophenol 95-95-4 400.0 D042 2,4,6-Trichlorophenol 88-06-2 2.0 D017 2,4,5-TP (Silvex) 93-72-1 1.0 D043 Vinyl Chloride 74-01-4 0.2 * Quantitation limit is greater than the calculated regulatory level. -

Toxicological Profile for 1,1-Dichloroethene Draft for Public Comment December 2019 1,1-DICHLOROETHENE Ii
F Toxicological Profile for 1,1-Dichloroethene Draft for Public Comment December 2019 1,1-DICHLOROETHENE ii DISCLAIMER Use of trade names is for identification only and does not imply endorsement by the Agency for Toxic Substances and Disease Registry, the Public Health Service, or the U.S. Department of Health and Human Services. This information is distributed solely for the purpose of pre dissemination public comment under applicable information quality guidelines. It has not been formally disseminated by the Agency for Toxic Substances and Disease Registry. It does not represent and should not be construed to represent any agency determination or policy. ***DRAFT FOR PUBLIC COMMENT*** 1,1-DICHLOROETHENE iii FOREWORD This toxicological profile is prepared in accordance with guidelines developed by the Agency for Toxic Substances and Disease Registry (ATSDR) and the Environmental Protection Agency (EPA). The original guidelines were published in the Federal Register on April 17, 1987. Each profile will be revised and republished as necessary. The ATSDR toxicological profile succinctly characterizes the toxicologic and adverse health effects information for these toxic substances described therein. Each peer-reviewed profile identifies and reviews the key literature that describes a substance's toxicologic properties. Other pertinent literature is also presented, but is described in less detail than the key studies. The profile is not intended to be an exhaustive document; however, more comprehensive sources of specialty information are referenced. The focus of the profiles is on health and toxicologic information; therefore, each toxicological profile begins with a relevance to public health discussion which would allow a public health professional to make a real-time determination of whether the presence of a particular substance in the environment poses a potential threat to human health. -

1,1-Dichloroethene (Vinylidene Chloride)
This report contains the collective views of an international group of experts and does not necessarily represent the decisions or the stated policy of the United Nations Environment Programme, the International Labour Organization, or the World Health Organization. Concise International Chemical Assessment Document 51 1,1-DICHLOROETHENE (VINYLIDENE CHLORIDE) Please note that the pagination and layout of this web version are not identical to the printed CICAD First draft prepared by Dr Bob Benson, US Environmental Protection Agency, Denver, Colorado, USA Published under the joint sponsorship of the United Nations Environment Programme, the International Labour Organization, and the World Health Organization, and produced within the framework of the Inter-Organization Programme for the Sound Management of Chemicals. World Health Organization Geneva, 2003 The International Programme on Chemical Safety (IPCS), established in 1980, is a joint venture of the United Nations Environment Programme (UNEP), the International Labour Organization (ILO), and the World Health Organization (WHO). The overall objectives of the IPCS are to establish the scientific basis for assessment of the risk to human health and the environment from exposure to chemicals, through international peer review processes, as a prerequisite for the promotion of chemical safety, and to provide technical assistance in strengthening national capacities for the sound management of chemicals. The Inter-Organization Programme for the Sound Management of Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food and Agriculture Organization of the United Nations, WHO, the United Nations Industrial Development Organization, the United Nations Institute for Training and Research, and the Organisation for Economic Co-operation and Development (Participating Organizations), following recommendations made by the 1992 UN Conference on Environment and Development to strengthen cooperation and increase coordination in the field of chemical safety. -

High Hazard Chemical Policy
Environmental Health & Safety Policy Manual Issue Date: 2/23/2011 Policy # EHS-200.09 High Hazard Chemical Policy 1.0 PURPOSE: To minimize hazardous exposures to high hazard chemicals which include select carcinogens, reproductive/developmental toxins, chemicals that have a high degree of toxicity. 2.0 SCOPE: The procedures provide guidance to all LSUHSC personnel who work with high hazard chemicals. 3.0 REPONSIBILITIES: 3.1 Environmental Health and Safety (EH&S) shall: • Provide technical assistance with the proper handling and safe disposal of high hazard chemicals. • Maintain a list of high hazard chemicals used at LSUHSC, see Appendix A. • Conduct exposure assessments and evaluate exposure control measures as necessary. Maintain employee exposure records. • Provide emergency response for chemical spills. 3.2 Principle Investigator (PI) /Supervisor shall: • Develop and implement a laboratory specific standard operation plan for high hazard chemical use per OSHA 29CFR 1910.1450 (e)(3)(i); Occupational Exposure to Hazardous Chemicals in Laboratories. • Notify EH&S of the addition of a high hazard chemical not previously used in the laboratory. • Ensure personnel are trained on specific chemical hazards present in the lab. • Maintain Material Safety Data Sheets (MSDS) for all chemicals, either on the computer hard drive or in hard copy. • Coordinate the provision of medical examinations, exposure monitoring and recordkeeping as required. 3.3 Employees: • Complete all necessary training before performing any work. • Observe all safety -

Differential Regulation of Endothelium Behavior by Progesterone and Medroxyprogesterone Acetate
P H CUTINI and others Progestins and vascular function 220:3 179–193 Research Differential regulation of endothelium behavior by progesterone and medroxyprogesterone acetate Pablo H Cutini1,2, Adria´n E Campelo1,2 and Virginia L Massheimer1,2 Correspondence should be addressed 1Ca´ tedra de Bioquı´mica Clı´nica II, Departamento de Biologı´a, Bioquı´mica y Farmacia, Universidad Nacional to V L Massheimer del Sur (UNS), San Juan 670, B8000ICN, Bahı´a Blanca, Argentina Email 2Consejo Nacional de Investigaciones Cientı´ficas y Te´ cnicas (CONICET), Argentina, Buenos Aires, Argentina [email protected] Abstract Medroxyprogesterone acetate (MPA) is a synthetic progestin commonly used in hormone Key Words replacement therapy (HRT). The aim of this research was to study and compare the effect of " cell migration progesterone (Pg) and MPA on the regulation of cellular events associated with vascular " medroxyprogesterone homeostasis and disease. Platelet adhesion to endothelial cells (ECs), nitric oxide (NO) acetate production, and cell migration were studied using murine ECs in vitro exposed to the " nitric oxide progestins. After 7 min of treatment, MPA significantly inhibited NO synthesis with respect " progesterone to control values; meanwhile, Pg markedly increased vasoactive production. In senile ECs, " vascular tissue the stimulatory action of Pg decreases; meanwhile, MPA maintained its ability to inhibit Journal of Endocrinology NO synthesis. The presence of RU486 antagonized the action of each steroid. When ECs were preincubated with PD98059 (MAPK inhibitor) or chelerythrine (protein kinase C (PKC) inhibitor) before Pg or MPA treatment, the former totally suppressed the steroid action, but the PKC antagonist did not affect NO production. -

Progesterone Using ALZET Osmotic Pumps
ALZET® Bibliography References on the Administration of Progesterone Using ALZET Osmotic Pumps Q8558: V. Joseph, et al. Progesterone decreases apnoea and reduces oxidative stress induced by chronic intermittent hypoxia in ovariectomized female rats. Exp Physiol 2020;105(6):1025-1034 Agents: Progesterone Vehicle: Cyclodextrin, 2-ß-Hydroxypropl-; Route: SC; Species: Rat; Pump: 2ML4; Duration: 28 days; ALZET Comments: Dose (4 mg/kg/day); Controls received mp w/ vehicle; animal info (Sprague-Dawley female rats (220-250g/57-70 days old)); post op. care (buprenorphine); Blood pressure measured via tail cuff method;93.3 mmHg - 105.2 mmHg;Progesterone aka prog; dependence; Q6232: S. F. Rosen, et al. T-Cell Mediation of Pregnancy Analgesia Affecting Chronic Pain in Mice. J Neurosci 2017;37(41):9819-9827 ALZET Comments: Estradiol, 17b-; Progesterone sulfate; SC; Mice; 2002; 14 days; Dose (17b-estradiol : 0.1 mg/kg/d, progesterone sulfate: 0.25 mg/kg/d, 0.1 mg/kg/d estradiol + 0.25 mg/kg/d progesterone); Controls received mp w/ vehicle; animal info (7-12 week old female C57BL/6J mice); replacement therapy (estradiol, ovariectomy); Therapeutic indication. Q6066: D. J. Morris, et al. Glucocorticoids and gut bacteria: "The GALF Hypothesis" in the metagenomic era. Steroids 2017;125(1-13 ALZET Comments: Chenodeoxycholic acid, progesterone, 11b-hydroxy-, corticosterone, deoxy-, corticosterone, 3α,5α-TH-, progesterone, 3α,5α-TH-11β-hydroxy-; SC; Rat; steroidal derivatives of corticosterone; Review presents the role of gut microbial metabolism of endogenous adrenocorticosteroids as a contributing factor in the etiology of essential hypertension. Q6204: S. McIlvride, et al. A progesterone-brown fat axis is involved in regulating fetal growth. -

Nitric Oxide Production, Inhibitory, Antioxidant and Antimycobacterial Activities of the Fruits Extract and Flavonoid Content of Schinus Terebinthifolius
Rev Bras Farmacogn 24(2014): 644-650 Original article Nitric oxide production, inhibitory, antioxidant and antimycobacterial activities of the fruits extract and flavonoid content of Schinus terebinthifolius Natalia R. Bernardesa, Marlon Heggdorne-Araújoa,b, Isabela F. J. C. Borgesb,c, Fabricio M. Almeidab, Eduardo P. Amaralb, Elena B. Lasunskaiab, Michelle F. Muzitanob,c,*, Daniela B. Oliveiraa,* aLaboratório de Tecnologia de Alimentos, Centro de Ciências e Tecnologias Agropecuárias, Universidade Estadual do Norte Fluminense Darcy Ribeiro, Campos dos Goytacazes, RJ, Brazil bLaboratório de Biologia do Reconhecer, Centro de Biociências e Biotecnologia, Universidade Estadual do Norte Fluminense Darcy Ribeiro, Campos dos Goytacazes, RJ, Brazil cLaboratório de Produtos Naturais, Curso de Farmácia, Universidade Federal do Rio de Janeiro, Campus Macaé, Polo Novo Cavaleiro, Instituto Macaé de Metrologia e Tecnologia, Macaé, RJ, Brazil ARTICLE INFO ABSTRACT Article history: The extract of the fruits from Schinus terebinthifolius Raddi, Anacardiaceae, was obtained by Received 28 May 2014 exhaustive extraction with methanol. Its fractions and isolated compounds were collected Accepted 16 October 2014 by fractionation with RP-2 column chromatography. The crude extract, the flavonoid frac- tion and the isolated compound identified as apigenin (1), were investigated regarding its Keywords: inhibitory action of nitric oxide production by LPS-stimulated macrophages, antioxidant Anacardiaceae activity by DPPH and the antimycobacterial activity against Mycobacterium bovis BCG. The Apigenin samples exhibited a significant inhibitory effect on the nitric oxide production (e.g., 1, IC50 Inflammation 19.23 ± 1.64 μg/ml) and also showed antioxidant activity. In addition, S. terebinthifolius sam- Mycobacterium ples inhibited the mycobacterial growth (e.g., 1, IC50 14.53 ± 1.25 μg/ml). -

A STUDY of the COMPOSITION and SOME REACTIONS OP the ETHERATE OP ALUMINUM TRIBTHYL DISSERTATION. Presented in Partial Ïtilfillm
A STUDY OF THE COMPOSITION AND SOME REACTIONS OP THE ETHERATE OP ALUMINUM TRIBTHYL DISSERTATION. Presented in Partial ïtilfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State ■ University ■ By EVELYN RUTH BLUST BAKER, B.A., 1.5, w The Ohio State University 1953 Approved by* Z’ f ' X ' / - y 1 ■■ ' . 7 ' ■------- 4 .dviser ACKNOWLEDGEMENT The author would like to thank Dr. Harry fl. Sisler for suggesting this study and for his continued interest and guidance throughout the course of the work. 933320 ii TABLE OF CONTENTS Page Chapter I* Introduction A» Purpose and Scope of this Research 1 B. Review of Methods of Preparation of Aluminum Triethyl and Aluminum Triethyl Ethorate 2 C. Properties Given in the Literature 4 Chapter II. Experimental Results and Discussion A. Experimental Methods and Precautions Employed in this Work 5 B. Preparation of Aluminum Triethyl and Aluminum Triethyl Etherate 8 0 . A Phase Study of the Binary System Aluminum Triethyl-Diethyl Ether 11 Do Reaction of Aluminum Triethyl Etherate with Alkali Metal Alkyls 24 E. Reaction of Aluminum Triethyl Etherate with Sulfur Dioxide 30 P. Reaction of Aluminum Triethyl Etherate with Nitrogen Dioxide 35 6 . Reaction of Aluminum Triethyl Etherate with Nitric Oxide 42 H. Reaction of Aluminum Triethyl Etherate with Pyridine 47 Ill I. Additional Reactions of Aluminum Triethyl Etherate* 1 . Reactionwith Carbon Dioxide 50 2. Reactionwith Oxygen 51 3 . Reaction with Sulfur Trioxide 53 4 * Reaction with Carbon Tetrachloride 54 Chapter III. Conclusions and Summary ^6 Bibliography 59 A STUDY OP THE COMPOSITION AND SOME REACTIONS OP THE ETHERATE OP ALUMINUM TRIETHYL CHAPTER I. -

EUROPEAN PHARMACOPOEIA 10.0 Index 1. General Notices
EUROPEAN PHARMACOPOEIA 10.0 Index 1. General notices......................................................................... 3 2.2.66. Detection and measurement of radioactivity........... 119 2.1. Apparatus ............................................................................. 15 2.2.7. Optical rotation................................................................ 26 2.1.1. Droppers ........................................................................... 15 2.2.8. Viscosity ............................................................................ 27 2.1.2. Comparative table of porosity of sintered-glass filters.. 15 2.2.9. Capillary viscometer method ......................................... 27 2.1.3. Ultraviolet ray lamps for analytical purposes............... 15 2.3. Identification...................................................................... 129 2.1.4. Sieves ................................................................................. 16 2.3.1. Identification reactions of ions and functional 2.1.5. Tubes for comparative tests ............................................ 17 groups ...................................................................................... 129 2.1.6. Gas detector tubes............................................................ 17 2.3.2. Identification of fatty oils by thin-layer 2.2. Physical and physico-chemical methods.......................... 21 chromatography...................................................................... 132 2.2.1. Clarity and degree of opalescence of