Managing Hazardous Materials Incidents
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nitric Oxide in Health and Disease of the Nervous System H-Y Yun1,2, VL Dawson1,3,4 and TM Dawson1,3
Molecular Psychiatry (1997) 2, 300–310 1997 Stockton Press All rights reserved 1359–4184/97 $12.00 PROGRESS Nitric oxide in health and disease of the nervous system H-Y Yun1,2, VL Dawson1,3,4 and TM Dawson1,3 Departments of 1Neurology; 3Neuroscience; 4Physiology, Johns Hopkins University School of Medicine, Baltimore, MD, USA Nitric oxide (NO) is a widespread and multifunctional biological messenger molecule. It mediates vasodilation of blood vessels, host defence against infectious agents and tumors, and neurotransmission of the central and peripheral nervous systems. In the nervous system, NO is generated by three nitric oxide synthase (NOS) isoforms (neuronal, endothelial and immunologic NOS). Endothelial NOS and neuronal NOS are constitutively expressed and acti- vated by elevated intracellular calcium, whereas immunologic NOS is inducible with new RNA and protein synthesis upon immune stimulation. Neuronal NOS can be transcriptionally induced under conditions such as neuronal development and injury. NO may play a role not only in physiologic neuronal functions such as neurotransmitter release, neural development, regeneration, synaptic plasticity and regulation of gene expression but also in a variety of neurological disorders in which excessive production of NO leads to neural injury. Keywords: nitric oxide synthase; endothelium-derived relaxing factor; neurotransmission; neurotoxic- ity; neurological diseases Nitric oxide is probably the smallest and most versatile NO synthases isoforms and regulation of NO bioactive molecule identified. Convergence of multi- generation disciplinary efforts in the field of immunology, cardio- vascular pharmacology, chemistry, toxicology and neu- NO is formed by the enzymatic conversion of the guan- robiology led to the revolutionary novel concept of NO idino nitrogen of l-arginine by NO synthase (NOS). -

Chapter 7.1 Nitrogen Dioxide
Chapter 7.1 Nitrogen dioxide General description Many chemical species of nitrogen oxides (NOx) exist, but the air pollutant species of most interest from the point of view of human health is nitrogen dioxide (NO2). Nitrogen dioxide is soluble in water, reddish-brown in colour, and a strong oxidant. Nitrogen dioxide is an important atmospheric trace gas, not only because of its health effects but also because (a) it absorbs visible solar radiation and contributes to impaired atmospheric visibility; (b) as an absorber of visible radiation it could have a potential direct role in global climate change if its concentrations were to become high enough; (c) it is, along with nitric oxide (NO), a chief regulator of the oxidizing capacity of the free troposphere by controlling the build-up and fate of radical species, including hydroxyl radicals; and (d) it plays a critical role in determining ozone (O3) concentrations in the troposphere because the photolysis of nitrogen dioxide is the only key initiator of the photochemical formation of ozone, whether in polluted or unpolluted atmospheres (1, 2). Sources On a global scale, emissions of nitrogen oxides from natural sources far outweigh those generated by human activities. Natural sources include intrusion of stratospheric nitrogen oxides, bacterial and volcanic action, and lightning. Because natural emissions are distributed over the entire surface of the earth, however, the resulting background atmospheric concentrations are very small. The major source of anthropogenic emissions of nitrogen oxides into the atmosphere is the combustion of fossil fuels in stationary sources (heating, power generation) and in motor vehicles (internal combustion engines). -

Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 11
This PDF is available from The National Academies Press at http://www.nap.edu/catalog.php?record_id=13374 Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 11 ISBN Committee on Acute Exposure Guideline Levels; Committee on 978-0-309-25481-6 Toxicology; National Research Council 356 pages 6 x 9 PAPERBACK (2012) Visit the National Academies Press online and register for... Instant access to free PDF downloads of titles from the NATIONAL ACADEMY OF SCIENCES NATIONAL ACADEMY OF ENGINEERING INSTITUTE OF MEDICINE NATIONAL RESEARCH COUNCIL 10% off print titles Custom notification of new releases in your field of interest Special offers and discounts Distribution, posting, or copying of this PDF is strictly prohibited without written permission of the National Academies Press. Unless otherwise indicated, all materials in this PDF are copyrighted by the National Academy of Sciences. Request reprint permission for this book Copyright © National Academy of Sciences. All rights reserved. Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 11 Committee on Acute Exposure Guideline Levels Committee on Toxicology Board on Environmental Studies and Toxicology Division on Earth and Life Studies Copyright © National Academy of Sciences. All rights reserved. Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 11 THE NATIONAL ACADEMIES PRESS 500 FIFTH STREET, NW WASHINGTON, DC 20001 NOTICE: The project that is the subject of this report was approved by the Governing Board of the National Research Council, whose members are drawn from the councils of the National Academy of Sciences, the National Academy of Engineering, and the Insti- tute of Medicine. The members of the committee responsible for the report were chosen for their special competences and with regard for appropriate balance. -

Nitrogen Oxides
Pollution Prevention and Abatement Handbook WORLD BANK GROUP Effective July 1998 Nitrogen Oxides Nitrogen oxides (NOx) in the ambient air consist 1994). The United States generates about 20 mil- primarily of nitric oxide (NO) and nitrogen di- lion metric tons of nitrogen oxides per year, about oxide (NO2). These two forms of gaseous nitro- 40% of which is emitted from mobile sources. Of gen oxides are significant pollutants of the lower the 11 million to 12 million metric tons of nitrogen atmosphere. Another form, nitrous oxide (N2O), oxides that originate from stationary sources, is a greenhouse gas. At the point of discharge about 30% is the result of fuel combustion in large from man-made sources, nitric oxide, a colorless, industrial furnaces and 70% is from electric utility tasteless gas, is the predominant form of nitro- furnaces (Cooper and Alley 1986). gen oxide. Nitric oxide is readily converted to the much more harmful nitrogen dioxide by Occurrence in Air and Routes of Exposure chemical reaction with ozone present in the at- mosphere. Nitrogen dioxide is a yellowish-or- Annual mean concentrations of nitrogen dioxide ange to reddish-brown gas with a pungent, in urban areas throughout the world are in the irritating odor, and it is a strong oxidant. A por- range of 20–90 micrograms per cubic meter (µg/ tion of nitrogen dioxide in the atmosphere is con- m3). Maximum half-hour values and maximum 24- verted to nitric acid (HNO3) and ammonium hour values of nitrogen dioxide can approach 850 salts. Nitrate aerosol (acid aerosol) is removed µg/m3 and 400 µg/m3, respectively. -

American War and Military Operations Casualties: Lists and Statistics
American War and Military Operations Casualties: Lists and Statistics Updated July 29, 2020 Congressional Research Service https://crsreports.congress.gov RL32492 American War and Military Operations Casualties: Lists and Statistics Summary This report provides U.S. war casualty statistics. It includes data tables containing the number of casualties among American military personnel who served in principal wars and combat operations from 1775 to the present. It also includes data on those wounded in action and information such as race and ethnicity, gender, branch of service, and cause of death. The tables are compiled from various Department of Defense (DOD) sources. Wars covered include the Revolutionary War, the War of 1812, the Mexican War, the Civil War, the Spanish-American War, World War I, World War II, the Korean War, the Vietnam Conflict, and the Persian Gulf War. Military operations covered include the Iranian Hostage Rescue Mission; Lebanon Peacekeeping; Urgent Fury in Grenada; Just Cause in Panama; Desert Shield and Desert Storm; Restore Hope in Somalia; Uphold Democracy in Haiti; Operation Enduring Freedom (OEF); Operation Iraqi Freedom (OIF); Operation New Dawn (OND); Operation Inherent Resolve (OIR); and Operation Freedom’s Sentinel (OFS). Starting with the Korean War and the more recent conflicts, this report includes additional detailed information on types of casualties and, when available, demographics. It also cites a number of resources for further information, including sources of historical statistics on active duty military deaths, published lists of military personnel killed in combat actions, data on demographic indicators among U.S. military personnel, related websites, and relevant CRS reports. Congressional Research Service American War and Military Operations Casualties: Lists and Statistics Contents Introduction .................................................................................................................................... -
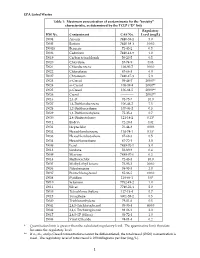
EPA Listed Wastes Table 1: Maximum Concentration of Contaminants For
EPA Listed Wastes Table 1: Maximum concentration of contaminants for the toxicity characteristic, as determined by the TCLP (D list) Regulatory HW No. Contaminant CAS No. Level (mg/L) D004 Arsenic 7440-38-2 5.0 D005 Barium 7440-39-3 100.0 D0018 Benzene 71-43-2 0.5 D006 Cadmium 7440-43-9 1.0 D019 Carbon tetrachloride 56-23-5 0.5 D020 Chlordane 57-74-9 0.03 D021 Chlorobenzene 108-90-7 100.0 D022 Chloroform 67-66-3 6.0 D007 Chromium 7440-47-3 5.0 D023 o-Cresol 95-48-7 200.0** D024 m-Cresol 108-39-4 200.0** D025 p-Cresol 106-44-5 200.0** D026 Cresol ------------ 200.0** D016 2,4-D 94-75-7 10.0 D027 1,4-Dichlorobenzene 106-46-7 7.5 D028 1,2-Dichloroethane 107-06-2 0.5 D029 1,1-Dichloroethylene 75-35-4 0.7 D030 2,4-Dinitrotoluene 121-14-2 0.13* D012 Endrin 72-20-8 0.02 D031 Heptachlor 76-44-8 0.008 D032 Hexachlorobenzene 118-74-1 0.13* D033 Hexachlorobutadiene 87-68-3 0.5 D034 Hexachloroethane 67-72-1 3.0 D008 Lead 7439-92-1 5.0 D013 Lindane 58-89-9 0.4 D009 Mercury 7439-97-6 0.2 D014 Methoxychlor 72-43-5 10.0 D035 Methyl ethyl ketone 78-93-3 200.0 D036 Nitrobenzene 98-95-3 2.0 D037 Pentachlorophenol 87-86-5 100.0 D038 Pyridine 110-86-1 5.0* D010 Selenium 7782-49-2 1.0 D011 Silver 7740-22-4 5.0 D039 Tetrachloroethylene 127-18-4 0.7 D015 Toxaphene 8001-35-2 0.5 D040 Trichloroethylene 79-01-6 0.5 D041 2,4,5-Trichlorophenol 95-95-4 400.0 D042 2,4,6-Trichlorophenol 88-06-2 2.0 D017 2,4,5-TP (Silvex) 93-72-1 1.0 D043 Vinyl Chloride 74-01-4 0.2 * Quantitation limit is greater than the calculated regulatory level. -

WHO Guidelines for Indoor Air Quality : Selected Pollutants
WHO GUIDELINES FOR INDOOR AIR QUALITY WHO GUIDELINES FOR INDOOR AIR QUALITY: WHO GUIDELINES FOR INDOOR AIR QUALITY: This book presents WHO guidelines for the protection of pub- lic health from risks due to a number of chemicals commonly present in indoor air. The substances considered in this review, i.e. benzene, carbon monoxide, formaldehyde, naphthalene, nitrogen dioxide, polycyclic aromatic hydrocarbons (especially benzo[a]pyrene), radon, trichloroethylene and tetrachloroethyl- ene, have indoor sources, are known in respect of their hazard- ousness to health and are often found indoors in concentrations of health concern. The guidelines are targeted at public health professionals involved in preventing health risks of environmen- SELECTED CHEMICALS SELECTED tal exposures, as well as specialists and authorities involved in the design and use of buildings, indoor materials and products. POLLUTANTS They provide a scientific basis for legally enforceable standards. World Health Organization Regional Offi ce for Europe Scherfi gsvej 8, DK-2100 Copenhagen Ø, Denmark Tel.: +45 39 17 17 17. Fax: +45 39 17 18 18 E-mail: [email protected] Web site: www.euro.who.int WHO guidelines for indoor air quality: selected pollutants The WHO European Centre for Environment and Health, Bonn Office, WHO Regional Office for Europe coordinated the development of these WHO guidelines. Keywords AIR POLLUTION, INDOOR - prevention and control AIR POLLUTANTS - adverse effects ORGANIC CHEMICALS ENVIRONMENTAL EXPOSURE - adverse effects GUIDELINES ISBN 978 92 890 0213 4 Address requests for publications of the WHO Regional Office for Europe to: Publications WHO Regional Office for Europe Scherfigsvej 8 DK-2100 Copenhagen Ø, Denmark Alternatively, complete an online request form for documentation, health information, or for per- mission to quote or translate, on the Regional Office web site (http://www.euro.who.int/pubrequest). -

MASS CASUALTY TRAUMA TRIAGE PARADIGMS and PITFALLS July 2019
1 Mass Casualty Trauma Triage - Paradigms and Pitfalls EXECUTIVE SUMMARY Emergency medical services (EMS) providers arrive on the scene of a mass casualty incident (MCI) and implement triage, moving green patients to a single area and grouping red and yellow patients using triage tape or tags. Patients are then transported to local hospitals according to their priority group. Tagged patients arrive at the hospital and are assessed and treated according to their priority. Though this triage process may not exactly describe your agency’s system, this traditional approach to MCIs is the model that has been used to train American EMS As a nation, we’ve got a lot providers for decades. Unfortunately—especially in of trailers with backboards mass violence incidents involving patients with time- and colored tape out there critical injuries and ongoing threats to responders and patients—this model may not be feasible and may result and that’s not what the focus in mis-triage and avoidable, outcome-altering delays of mass casualty response is in care. Further, many hospitals have not trained or about anymore. exercised triage or re-triage of exceedingly large numbers of patients, nor practiced a formalized secondary triage Dr. Edward Racht process that prioritizes patients for operative intervention American Medical Response or transfer to other facilities. The focus of this paper is to alert EMS medical directors and EMS systems planners and hospital emergency planners to key differences between “conventional” MCIs and mass violence events when: • the scene is dynamic, • the number of patients far exceeds usual resources; and • usual triage and treatment paradigms may fail. -

Nitrogen Dioxide
Common Name: NITROGEN DIOXIDE CAS Number: 10102-44-0 RTK Substance number: 1376 DOT Number: UN 1067 Date: May 1989 Revision: April 2000 ----------------------------------------------------------------------- ----------------------------------------------------------------------- HAZARD SUMMARY * Nitrogen Dioxide can affect you when breathed in. * If you think you are experiencing any work-related health * Nitrogen Dioxide may cause mutations. Handle with problems, see a doctor trained to recognize occupational extreme caution. diseases. Take this Fact Sheet with you. * Contact can irritate and burn the skin and eyes with * Exposure to hazardous substances should be routinely possible eye damage. evaluated. This may include collecting personal and area * Breathing Nitrogen Dioxide can irritate the nose and air samples. You can obtain copies of sampling results throat. from your employer. You have a legal right to this * Breathing Nitrogen Dioxide can irritate the lungs causing information under OSHA 1910.1020. coughing and/or shortness of breath. Higher exposures can cause a build-up of fluid in the lungs (pulmonary edema), a medical emergency, with severe shortness of WORKPLACE EXPOSURE LIMITS breath. OSHA: The legal airborne permissible exposure limit * High levels can interfere with the ability of the blood to (PEL) is 5 ppm, not to be exceeded at any time. carry Oxygen causing headache, fatigue, dizziness, and a blue color to the skin and lips (methemoglobinemia). NIOSH: The recommended airborne exposure limit is Higher levels can cause trouble breathing, collapse and 1 ppm, which should not be exceeded at any even death. time. * Repeated exposure to high levels may lead to permanent lung damage. ACGIH: The recommended airborne exposure limit is 3 ppm averaged over an 8-hour workshift and IDENTIFICATION 5 ppm as a STEL (short term exposure limit). -

Afghanistan Index Also Including Selected Data on Pakistan
Afghanistan Index Also including selected data on Pakistan Ian S. Livingston and Michael O’Hanlon October 31, 2012 Brookings Tracks Reconstruction and Security in Afghanistan, Iraq and Pakistan Afghanistan Index » http://www.brookings.edu/afghanistanindex Iraq Index » http://www.brookings.edu/iraqindex TABLE OF CONTENTS 1 Security Indicators 1.1 American Troops Deployed to Afghanistan UPDATED 9.30.12 4 1.2 Other Foreign Troops Deployed to Afghanistan UPDATED 10.31.12 5 1.3 Troops Committed to NATO’s International Security Assistance Mission (ISAF) by Country UPDATED 10.31.12 5 1.4 Size of Afghan Security Forces on Duty, 2003-2012 6 1.5 Afghan Local Police Growth 6 1.6 Afghan Army Units Partnered with NATO Units 7 1.7 Attrition Rates among Select Afghan National Security Forces 7 1.8 Afghan National Army Basic Rifle Marksmanship Qualification 7 1.9 Afghan National Army and Afghan National Police Ethnicity 8 1.10 Assessment Levels of Afghan National Security Forces 8 1.11 Total Number of Private DoD Contractors in Afghanistan, 2007 through 2011 9 1.12 Number of Private Security Contractors in Afghanistan, 2007 through 2011 9 1.13 U.S. Government Civilians in Afghanistan, August 2008-2011 9 1.14 Number of Insurgent Attacks, 2008-2012 UPDATED 10.31.12 10 1.15 Attacks by Afghan Security Forces against Allied Troops UPDATED 10.31.12 10 1.16 U.S. and Coalition Troop Fatalities since October 7, 2001 UPDATED 10.31.12 11 1.17 Cause of Death for U.S. Troops UPDATED 10.31.12 11 1.18 Non-US Coalition Troop Fatalities by Country since October 2001 UPDATED 10.31.12 12 1.19 Proportion of Annual U.S. -

United States District Court Eastern District of Louisiana
Case 2:17-cv-05967-JCZ-JVM Document 30 Filed 01/05/18 Page 1 of 12 UNITED STATES DISTRICT COURT EASTERN DISTRICT OF LOUISIANA REPUBLIC FIRE AND CASUALTY CIVIL ACTION INSURANCE COMPANY VERSUS NO. 17-5967 MARDECHRIA CHARLES AND SECTION A (1) DERRICK MCDONALD ORDER AND REASONS Before the Court is a Motion to Dismiss Defendants’ Malicious Prosecution Counterclaim (Rec. Doc. 23) filed by Plaintiff/Counterdefendant Republic Fire and Casualty Insurance Company (“Republic”). Defendants/Counterclaimants Mardechria Charles and Derrick McDonald oppose this motion (collectively referred to as “Counterclaimants”). (Rec. Doc. 28). The motion, set for submission on November 15, 2017, is before the Court on the briefs without oral argument. This matter is set to be tried to a jury beginning on May 21, 2018 at 8:30 a.m. Republic seeks dismissal of Counterclaimants’ claim pursuant to Federal Rule of Civil Procedure 12(b)(6) on the ground that Counterclaimants have failed to state a “plausible” claim for relief. Having considered the motion and memoranda of counsel, the record, and the applicable law, the Court finds that Republic’s motion should be GRANTED for the reasons set forth below. I. Background This matter arises out of a house fire that occurred in Vacherie, Louisiana at a home owned by Mardechria Charles. On June 20, 2017, Republic—insurer of the home—brought suit against Ms. Charles and her husband Derrick McDonald seeking declaratory judgment and restitution. Republic’s Complaint seeks a judicial declaration that Republic owes no further payments under Case 2:17-cv-05967-JCZ-JVM Document 30 Filed 01/05/18 Page 2 of 12 the subject policy of insurance and that Republic is entitled to reimbursement of the payments it has made on behalf of Ms. -

James Hillier
14 City Lofts 112-116 Tabernacle Street London EC2A 4LE offi[email protected] +44 (0) 20 7734 6441 JAMES HILLIER Shadow & Bone Small Axe The Crown Television Role Title Production Company Director DCI Bill Raynott STEPHEN Hat Trick for ITV Alrick Riley Tony Leech DECEIT Story Films Niall MacCormack Jack Cocker CLOSE TO ME Viaplay / Channel 4 Michael Samuels Captain Churik SHADOW & BONE 21 Laps Entertainment / Netflix Lee Toland Krieger / Eric Heisserer Chief Inspector SMALL AXE BBC / Amazon Studios Steve McQueen Dr Stu Ford DOCTORS BBC Dan Wilson The Equerry (Series Regular) THE CROWN SEASON TWO Left Bank Pictures / Netflix Stephen Daldry Nathan Stone PRIME SUSPECT 1973 Noho / ITV David Caffrey The Equerry (Series Regular) THE CROWN SEASON ONE TVE Various Oliver Grau (Series Regular) MERLÍ SEASON 1 Left Bank Pictures / Netflix Stephen Daldry Joseph McCoy FRONTIER Raw TV Ben Chanan James Downing CASUALTY BBC Jon Sen Admiral Nelson THE BRITISH Nutopia Jenny Ash Chris LONDON’S BURNING Juniper Justin Hardy Mick SURVIVORS BBC Ian B. McDonald Sgt Christian Young (Series HOLBY BLUE SERIES TWO BBC / Kudos Martin Hutchins Regular) Damian EASTENDERS BBC Michael Kellior Sgt Christian Young (Series HOLBY BLUE SERIES ONE BBC / Kudos Martin Hutchins Regular) Keith Spalding GOLDPLATED Channel 4 Julie Ann Robinson / Robert Delamere Robert Barrie (Recurring) THE BILL Talkback Thames Bill Scot-Rider Marcus Octavius THE RISE AND FALL OF ROME: BBC Chris Spencer REVOLUTION Garret Gibbens BLACKBEARD Dangerous Films Richard Dale Darren HOLBY CITY BBC Nick Adams Jeremy