Nitric Oxide
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nitric Oxide in Health and Disease of the Nervous System H-Y Yun1,2, VL Dawson1,3,4 and TM Dawson1,3
Molecular Psychiatry (1997) 2, 300–310 1997 Stockton Press All rights reserved 1359–4184/97 $12.00 PROGRESS Nitric oxide in health and disease of the nervous system H-Y Yun1,2, VL Dawson1,3,4 and TM Dawson1,3 Departments of 1Neurology; 3Neuroscience; 4Physiology, Johns Hopkins University School of Medicine, Baltimore, MD, USA Nitric oxide (NO) is a widespread and multifunctional biological messenger molecule. It mediates vasodilation of blood vessels, host defence against infectious agents and tumors, and neurotransmission of the central and peripheral nervous systems. In the nervous system, NO is generated by three nitric oxide synthase (NOS) isoforms (neuronal, endothelial and immunologic NOS). Endothelial NOS and neuronal NOS are constitutively expressed and acti- vated by elevated intracellular calcium, whereas immunologic NOS is inducible with new RNA and protein synthesis upon immune stimulation. Neuronal NOS can be transcriptionally induced under conditions such as neuronal development and injury. NO may play a role not only in physiologic neuronal functions such as neurotransmitter release, neural development, regeneration, synaptic plasticity and regulation of gene expression but also in a variety of neurological disorders in which excessive production of NO leads to neural injury. Keywords: nitric oxide synthase; endothelium-derived relaxing factor; neurotransmission; neurotoxic- ity; neurological diseases Nitric oxide is probably the smallest and most versatile NO synthases isoforms and regulation of NO bioactive molecule identified. Convergence of multi- generation disciplinary efforts in the field of immunology, cardio- vascular pharmacology, chemistry, toxicology and neu- NO is formed by the enzymatic conversion of the guan- robiology led to the revolutionary novel concept of NO idino nitrogen of l-arginine by NO synthase (NOS). -

2016 Bill No. CS for SB 1528 Ì460300XÎ460300
Florida Senate - 2016 PROPOSED COMMITTEE SUBSTITUTE Bill No. CS for SB 1528 460300 Ì460300XÎ 576-03397-16 Proposed Committee Substitute by the Committee on Appropriations (Appropriations Subcommittee on Criminal and Civil Justice) 1 A bill to be entitled 2 An act relating to illicit drugs; amending s. 893.02, 3 F.S.; defining terms; deleting a definition; revising 4 definitions; amending s. 893.03, F.S.; providing that 5 class designation is a way to reference scheduled 6 controlled substances; adding, deleting, and revising 7 the list of Schedule I controlled substances; revising 8 the list of Schedule III anabolic steroids; amending 9 s. 893.033, F.S.; adding, deleting, and revising the 10 list of precursor and essential chemicals; amending s. 11 893.0356, F.S.; defining the term “substantially 12 similar”; deleting the term “potential for abuse”; 13 requiring that a controlled substance analog be 14 treated as the highest scheduled controlled substance 15 of which it is an analog; amending s. 893.13, F.S.; 16 creating a noncriminal penalty for selling, 17 manufacturing, or delivering, or possessing with 18 intent to sell, manufacture, or deliver any unlawful 19 controlled substance in, on, or near an assisted 20 living facility; creating a criminal penalty for a 21 person 18 years of age or older who delivers to a 22 person younger than 18 years of age any illegal 23 controlled substance, who uses or hires a person 24 younger than 18 years of age in the sale or delivery 25 of such substance, or who uses a person younger than 26 18 years of age to assist in avoiding detection for 27 specified violations; deleting a criminal penalty for Page 1 of 197 2/12/2016 8:29:32 AM Florida Senate - 2016 PROPOSED COMMITTEE SUBSTITUTE Bill No. -

Unesco – Eolss Sample Chapters
PHARMACOLOGY – Vol. II - Anesthetics - Amanda Baric and David Pescod. ANESTHETICS Amanda Baric and David Pescod. Department of Anesthesia and Perioperative medicine, The Northern Hospital, Melbourne, Australia. Keywords: Anesthesia, pharmacology, inhalational, neuromuscular blockade, induction agent, local anesthetic. Contents 1. Inhalation agents 1.1. Introduction 1.2. Pharmacokinetics and Pharmacodynamics 1.3. Specific Agents 1.3.1. Diethyl Ether (Ether) 1.3.2. Chloroform 1.3.3. Cyclopropane 1.3.4. Trichloroethylene 1.3.5. Halogenated Alkanes and Ethers 1.3.6. Nitrous Oxide. 1.3.7. Xenon 2. Neuromuscular blocking agents. 2.1. Introduction 2.2. Non-Depolarizing Muscle Relaxants 2.2.1. Tubocurarine (1935) 2.2.2. Metocurine (dimethyl tubocurarine chloride/bromide) 2.2.3. Alcuronium (1961) 2.2.4. Gallamine (1948) 2.2.5. Pancuronium (1968) 2.2.6. Vecuronium (1983) 2.2.7. Atracurium (1980s) 2.2.8. Cis-atracurium (1995) 2.2.9. Mivacurium (1993) 2.2.10. Rocuronium (1994) 2.2.11. SugammadexUNESCO (2003) – EOLSS 2.2.12. Rapacuronium 2.3. Reversal Drugs (Anticholinesterase) 2.4. DepolarizingSAMPLE Muscle Relaxants (Suxamethonium CHAPTERS or Succinylcholine) 3. Local anesthetics 3.1. Introduction 3.2. Pharmacokinetics and Pharmacodynamics 3.3. Toxicity 3.4. Specific Agents 3.4.1. Cocaine 3.4.2. Procaine 3.4.3 Chloroprocaine 3.4.4. Tetracaine (Amethocaine) ©Encyclopedia of Life Support Systems (EOLSS) PHARMACOLOGY – Vol. II - Anesthetics - Amanda Baric and David Pescod. 3.4.5. Lidocaine 3.4.6. Prilocaine 3.4.7. Mepivacaine 3.4.8. Bupivacaine 3.4.9. Ropivacaine 3.4.10. Eutectic Mixture of Local Anesthetics (EMLA) 4. Intravenous Induction Agents 4.1. -

Pharmacology – Inhalant Anesthetics
Pharmacology- Inhalant Anesthetics Lyon Lee DVM PhD DACVA Introduction • Maintenance of general anesthesia is primarily carried out using inhalation anesthetics, although intravenous anesthetics may be used for short procedures. • Inhalation anesthetics provide quicker changes of anesthetic depth than injectable anesthetics, and reversal of central nervous depression is more readily achieved, explaining for its popularity in prolonged anesthesia (less risk of overdosing, less accumulation and quicker recovery) (see table 1) Table 1. Comparison of inhalant and injectable anesthetics Inhalant Technique Injectable Technique Expensive Equipment Cheap (needles, syringes) Patent Airway and high O2 Not necessarily Better control of anesthetic depth Once given, suffer the consequences Ease of elimination (ventilation) Only through metabolism & Excretion Pollution No • Commonly administered inhalant anesthetics include volatile liquids such as isoflurane, halothane, sevoflurane and desflurane, and inorganic gas, nitrous oxide (N2O). Except N2O, these volatile anesthetics are chemically ‘halogenated hydrocarbons’ and all are closely related. • Physical characteristics of volatile anesthetics govern their clinical effects and practicality associated with their use. Table 2. Physical characteristics of some volatile anesthetic agents. (MAC is for man) Name partition coefficient. boiling point MAC % blood /gas oil/gas (deg=C) Nitrous oxide 0.47 1.4 -89 105 Cyclopropane 0.55 11.5 -34 9.2 Halothane 2.4 220 50.2 0.75 Methoxyflurane 11.0 950 104.7 0.2 Enflurane 1.9 98 56.5 1.68 Isoflurane 1.4 97 48.5 1.15 Sevoflurane 0.6 53 58.5 2.5 Desflurane 0.42 18.7 25 5.72 Diethyl ether 12 65 34.6 1.92 Chloroform 8 400 61.2 0.77 Trichloroethylene 9 714 86.7 0.23 • The volatile anesthetics are administered as vapors after their evaporization in devices known as vaporizers. -
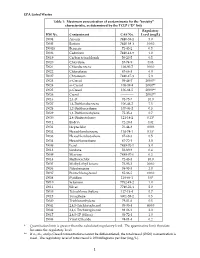
EPA Listed Wastes Table 1: Maximum Concentration of Contaminants For
EPA Listed Wastes Table 1: Maximum concentration of contaminants for the toxicity characteristic, as determined by the TCLP (D list) Regulatory HW No. Contaminant CAS No. Level (mg/L) D004 Arsenic 7440-38-2 5.0 D005 Barium 7440-39-3 100.0 D0018 Benzene 71-43-2 0.5 D006 Cadmium 7440-43-9 1.0 D019 Carbon tetrachloride 56-23-5 0.5 D020 Chlordane 57-74-9 0.03 D021 Chlorobenzene 108-90-7 100.0 D022 Chloroform 67-66-3 6.0 D007 Chromium 7440-47-3 5.0 D023 o-Cresol 95-48-7 200.0** D024 m-Cresol 108-39-4 200.0** D025 p-Cresol 106-44-5 200.0** D026 Cresol ------------ 200.0** D016 2,4-D 94-75-7 10.0 D027 1,4-Dichlorobenzene 106-46-7 7.5 D028 1,2-Dichloroethane 107-06-2 0.5 D029 1,1-Dichloroethylene 75-35-4 0.7 D030 2,4-Dinitrotoluene 121-14-2 0.13* D012 Endrin 72-20-8 0.02 D031 Heptachlor 76-44-8 0.008 D032 Hexachlorobenzene 118-74-1 0.13* D033 Hexachlorobutadiene 87-68-3 0.5 D034 Hexachloroethane 67-72-1 3.0 D008 Lead 7439-92-1 5.0 D013 Lindane 58-89-9 0.4 D009 Mercury 7439-97-6 0.2 D014 Methoxychlor 72-43-5 10.0 D035 Methyl ethyl ketone 78-93-3 200.0 D036 Nitrobenzene 98-95-3 2.0 D037 Pentachlorophenol 87-86-5 100.0 D038 Pyridine 110-86-1 5.0* D010 Selenium 7782-49-2 1.0 D011 Silver 7740-22-4 5.0 D039 Tetrachloroethylene 127-18-4 0.7 D015 Toxaphene 8001-35-2 0.5 D040 Trichloroethylene 79-01-6 0.5 D041 2,4,5-Trichlorophenol 95-95-4 400.0 D042 2,4,6-Trichlorophenol 88-06-2 2.0 D017 2,4,5-TP (Silvex) 93-72-1 1.0 D043 Vinyl Chloride 74-01-4 0.2 * Quantitation limit is greater than the calculated regulatory level. -

Zebrafish Behavioural Profiling Identifies GABA and Serotonin
ARTICLE https://doi.org/10.1038/s41467-019-11936-w OPEN Zebrafish behavioural profiling identifies GABA and serotonin receptor ligands related to sedation and paradoxical excitation Matthew N. McCarroll1,11, Leo Gendelev1,11, Reid Kinser1, Jack Taylor 1, Giancarlo Bruni 2,3, Douglas Myers-Turnbull 1, Cole Helsell1, Amanda Carbajal4, Capria Rinaldi1, Hye Jin Kang5, Jung Ho Gong6, Jason K. Sello6, Susumu Tomita7, Randall T. Peterson2,10, Michael J. Keiser 1,8 & David Kokel1,9 1234567890():,; Anesthetics are generally associated with sedation, but some anesthetics can also increase brain and motor activity—a phenomenon known as paradoxical excitation. Previous studies have identified GABAA receptors as the primary targets of most anesthetic drugs, but how these compounds produce paradoxical excitation is poorly understood. To identify and understand such compounds, we applied a behavior-based drug profiling approach. Here, we show that a subset of central nervous system depressants cause paradoxical excitation in zebrafish. Using this behavior as a readout, we screened thousands of compounds and identified dozens of hits that caused paradoxical excitation. Many hit compounds modulated human GABAA receptors, while others appeared to modulate different neuronal targets, including the human serotonin-6 receptor. Ligands at these receptors generally decreased neuronal activity, but paradoxically increased activity in the caudal hindbrain. Together, these studies identify ligands, targets, and neurons affecting sedation and paradoxical excitation in vivo in zebrafish. 1 Institute for Neurodegenerative Diseases, University of California, San Francisco, CA 94143, USA. 2 Cardiovascular Research Center and Division of Cardiology, Department of Medicine, Massachusetts General Hospital, Harvard Medical School, Charlestown, MA 02129, USA. -

(12) United States Patent (10) Patent N0.: US 6,310,270 B1 Huang Et Al
US006310270B1 (12) United States Patent (10) Patent N0.: US 6,310,270 B1 Huang et al. (45) Date of Patent: Oct. 30, 2001 (54) ENDOTHELIAL NOS KNOCKOUT MICE Benrath, J. et al., “Substance P and nitric oxide mediate AND METHODS OF USE Wound healing of ultraviolet photodamaged rat skin: evi dence for an effect of nitric oxide on keratinocyte prolifera (75) Inventors: Paul L. Huang, Boston; Mark C. tion,” Nerosci. Letts. 200.'17—20 (Nov. 1995). Fishman, Newton Center; Michael A. Boeckxstaens, G.E. et al., “Evidence for nitric oxide as Moskowitz, Belmont, all of MA (US) mediator of non—adrenergic, non—cholinergic relaxations induced by ATP and GABA in the canine gut,” Br J. (73) Assignee: The General Hospital Corporation, Pharmacol. 102:434—438 (1991). Boston, MA (US) Bohme, G.A. et al., “Possible involvement of nitric oxide in ( * ) Notice: Subject to any disclaimer, the term of this long—term potentiation,” Eur J. Pharmacol. 199:379—381 patent is extended or adjusted under 35 (1991). U.S.C. 154(b) by 0 days. Booth, R.F.G. et al., “Rapid development of atherosclerotic lesions in the rabbit carotid artery induced by perivascular (21) Appl. No.: 08/818,082 manipulation,” Atherosclerosis 76.'257—268 (1989). Bredt, D.S. et al., “Localization of nitric oxide synthase (22) Filed: Mar. 14, 1997 indicating a neural role for nitric oxide,” Nature Related US. Application Data 347:768—770. (60) Provisional application No. 60/027,362, ?led on Sep. 18, Bredt, D.S. and Snyder, S.H., “Isolation of nitric oxide 1996, and provisional application No. -

Pharmacokinetics and Pharmacology of Drugs Used in Children
Drug and Fluid Th erapy SECTION II Pharmacokinetics and Pharmacology of Drugs Used CHAPTER 6 in Children Charles J. Coté, Jerrold Lerman, Robert M. Ward, Ralph A. Lugo, and Nishan Goudsouzian Drug Distribution Propofol Protein Binding Ketamine Body Composition Etomidate Metabolism and Excretion Muscle Relaxants Hepatic Blood Flow Succinylcholine Renal Excretion Intermediate-Acting Nondepolarizing Relaxants Pharmacokinetic Principles and Calculations Atracurium First-Order Kinetics Cisatracurium Half-Life Vecuronium First-Order Single-Compartment Kinetics Rocuronium First-Order Multiple-Compartment Kinetics Clinical Implications When Using Short- and Zero-Order Kinetics Intermediate-Acting Relaxants Apparent Volume of Distribution Long-Acting Nondepolarizing Relaxants Repetitive Dosing and Drug Accumulation Pancuronium Steady State Antagonism of Muscle Relaxants Loading Dose General Principles Central Nervous System Effects Suggamadex The Drug Approval Process, the Package Insert, and Relaxants in Special Situations Drug Labeling Opioids Inhalation Anesthetic Agents Morphine Physicochemical Properties Meperidine Pharmacokinetics of Inhaled Anesthetics Hydromorphone Pharmacodynamics of Inhaled Anesthetics Oxycodone Clinical Effects Methadone Nitrous Oxide Fentanyl Environmental Impact Alfentanil Oxygen Sufentanil Intravenous Anesthetic Agents Remifentanil Barbiturates Butorphanol and Nalbuphine 89 A Practice of Anesthesia for Infants and Children Codeine Antiemetics Tramadol Metoclopramide Nonsteroidal Anti-infl ammatory Agents 5-Hydroxytryptamine -

Differential Regulation of Endothelium Behavior by Progesterone and Medroxyprogesterone Acetate
P H CUTINI and others Progestins and vascular function 220:3 179–193 Research Differential regulation of endothelium behavior by progesterone and medroxyprogesterone acetate Pablo H Cutini1,2, Adria´n E Campelo1,2 and Virginia L Massheimer1,2 Correspondence should be addressed 1Ca´ tedra de Bioquı´mica Clı´nica II, Departamento de Biologı´a, Bioquı´mica y Farmacia, Universidad Nacional to V L Massheimer del Sur (UNS), San Juan 670, B8000ICN, Bahı´a Blanca, Argentina Email 2Consejo Nacional de Investigaciones Cientı´ficas y Te´ cnicas (CONICET), Argentina, Buenos Aires, Argentina [email protected] Abstract Medroxyprogesterone acetate (MPA) is a synthetic progestin commonly used in hormone Key Words replacement therapy (HRT). The aim of this research was to study and compare the effect of " cell migration progesterone (Pg) and MPA on the regulation of cellular events associated with vascular " medroxyprogesterone homeostasis and disease. Platelet adhesion to endothelial cells (ECs), nitric oxide (NO) acetate production, and cell migration were studied using murine ECs in vitro exposed to the " nitric oxide progestins. After 7 min of treatment, MPA significantly inhibited NO synthesis with respect " progesterone to control values; meanwhile, Pg markedly increased vasoactive production. In senile ECs, " vascular tissue the stimulatory action of Pg decreases; meanwhile, MPA maintained its ability to inhibit Journal of Endocrinology NO synthesis. The presence of RU486 antagonized the action of each steroid. When ECs were preincubated with PD98059 (MAPK inhibitor) or chelerythrine (protein kinase C (PKC) inhibitor) before Pg or MPA treatment, the former totally suppressed the steroid action, but the PKC antagonist did not affect NO production. -

Dcanesthesiamanualvo
ii Any or all parts of this manual may be reproduced, provided the parts reproduced are free- not for sale. For commercial purposes, no part of this manual may be reproduced or utilized in any form or by any means, electronic or mechanical, including photocopying and recording, or by any information storage and retrieval system, without permission in writing from the publisher. The intent of this manual is to be freely used, copied, and distributed in Developing Countries for the teaching and promotion of basic anesthesia knowledge/skills. The purpose of this manual is to provide developing countries with a copyright free basic anesthesia manual. This manual can be freely copied and translated into a native language for the promotion of basic anesthesia knowledge/skills. Contributors with credited pictures and illustrations have graciously given permission for their material to be used for this specific purpose. The author and publishers of this manual cannot accept liability from the use of this manual or errors in translation. It is up to each translator to ensure that the translation is correct. Knowledge about the art and science of anesthesia continues to change. It is up to each anesthesia provider to continue to learn and upgrade their knowledge. This manual only contains basic knowledge and is not a replacement for more comprehensive anesthesia information. iii “Every prudent man acts out of knowledge.” Proverbs 13:15 Soli Deo Gloria iv Acknowledgements This project would not have been possible without the help of many. The World Health Organization and Michael B. Dobson MD kindly gave permission to utilize illustrations from the publication Anaesthesia at the District Hospital, WHO, Geneva, 2000 for two earlier editions, published in Afghanistan and Cambodia. -

Progesterone Using ALZET Osmotic Pumps
ALZET® Bibliography References on the Administration of Progesterone Using ALZET Osmotic Pumps Q8558: V. Joseph, et al. Progesterone decreases apnoea and reduces oxidative stress induced by chronic intermittent hypoxia in ovariectomized female rats. Exp Physiol 2020;105(6):1025-1034 Agents: Progesterone Vehicle: Cyclodextrin, 2-ß-Hydroxypropl-; Route: SC; Species: Rat; Pump: 2ML4; Duration: 28 days; ALZET Comments: Dose (4 mg/kg/day); Controls received mp w/ vehicle; animal info (Sprague-Dawley female rats (220-250g/57-70 days old)); post op. care (buprenorphine); Blood pressure measured via tail cuff method;93.3 mmHg - 105.2 mmHg;Progesterone aka prog; dependence; Q6232: S. F. Rosen, et al. T-Cell Mediation of Pregnancy Analgesia Affecting Chronic Pain in Mice. J Neurosci 2017;37(41):9819-9827 ALZET Comments: Estradiol, 17b-; Progesterone sulfate; SC; Mice; 2002; 14 days; Dose (17b-estradiol : 0.1 mg/kg/d, progesterone sulfate: 0.25 mg/kg/d, 0.1 mg/kg/d estradiol + 0.25 mg/kg/d progesterone); Controls received mp w/ vehicle; animal info (7-12 week old female C57BL/6J mice); replacement therapy (estradiol, ovariectomy); Therapeutic indication. Q6066: D. J. Morris, et al. Glucocorticoids and gut bacteria: "The GALF Hypothesis" in the metagenomic era. Steroids 2017;125(1-13 ALZET Comments: Chenodeoxycholic acid, progesterone, 11b-hydroxy-, corticosterone, deoxy-, corticosterone, 3α,5α-TH-, progesterone, 3α,5α-TH-11β-hydroxy-; SC; Rat; steroidal derivatives of corticosterone; Review presents the role of gut microbial metabolism of endogenous adrenocorticosteroids as a contributing factor in the etiology of essential hypertension. Q6204: S. McIlvride, et al. A progesterone-brown fat axis is involved in regulating fetal growth. -

Nitric Oxide Synthase Inhibitors As Antidepressants
Pharmaceuticals 2010, 3, 273-299; doi:10.3390/ph3010273 OPEN ACCESS pharmaceuticals ISSN 1424-8247 www.mdpi.com/journal/pharmaceuticals Review Nitric Oxide Synthase Inhibitors as Antidepressants Gregers Wegener 1,* and Vallo Volke 2 1 Centre for Psychiatric Research, University of Aarhus, Skovagervej 2, DK-8240 Risskov, Denmark 2 Department of Physiology, University of Tartu, Ravila 19, EE-70111 Tartu, Estonia; E-Mail: [email protected] (V.V.) * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +4577893524; Fax: +4577893549. Received: 10 November 2009; in revised form: 7 January 2010 / Accepted: 19 January 2010 / Published: 20 January 2010 Abstract: Affective and anxiety disorders are widely distributed disorders with severe social and economic effects. Evidence is emphatic that effective treatment helps to restore function and quality of life. Due to the action of most modern antidepressant drugs, serotonergic mechanisms have traditionally been suggested to play major roles in the pathophysiology of mood and stress-related disorders. However, a few clinical and several pre-clinical studies, strongly suggest involvement of the nitric oxide (NO) signaling pathway in these disorders. Moreover, several of the conventional neurotransmitters, including serotonin, glutamate and GABA, are intimately regulated by NO, and distinct classes of antidepressants have been found to modulate the hippocampal NO level in vivo. The NO system is therefore a potential target for antidepressant and anxiolytic drug action in acute therapy as well as in prophylaxis. This paper reviews the effect of drugs modulating NO synthesis in anxiety and depression. Keywords: nitric oxide; antidepressants; psychiatry; depression; anxiety 1. Introduction Recent data from Denmark and Europe [1,2], indicate that brain disorders account for 12% of all direct costs in the Danish health system and 9% of the total drug consumption was used for treatment of brain diseases.