The Influence of Posture and Brain Size on Foramen Magnum Position in Bats
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
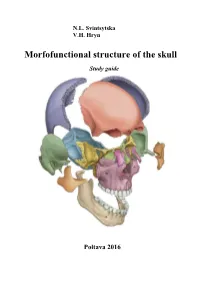
Morfofunctional Structure of the Skull
N.L. Svintsytska V.H. Hryn Morfofunctional structure of the skull Study guide Poltava 2016 Ministry of Public Health of Ukraine Public Institution «Central Methodological Office for Higher Medical Education of MPH of Ukraine» Higher State Educational Establishment of Ukraine «Ukranian Medical Stomatological Academy» N.L. Svintsytska, V.H. Hryn Morfofunctional structure of the skull Study guide Poltava 2016 2 LBC 28.706 UDC 611.714/716 S 24 «Recommended by the Ministry of Health of Ukraine as textbook for English- speaking students of higher educational institutions of the MPH of Ukraine» (minutes of the meeting of the Commission for the organization of training and methodical literature for the persons enrolled in higher medical (pharmaceutical) educational establishments of postgraduate education MPH of Ukraine, from 02.06.2016 №2). Letter of the MPH of Ukraine of 11.07.2016 № 08.01-30/17321 Composed by: N.L. Svintsytska, Associate Professor at the Department of Human Anatomy of Higher State Educational Establishment of Ukraine «Ukrainian Medical Stomatological Academy», PhD in Medicine, Associate Professor V.H. Hryn, Associate Professor at the Department of Human Anatomy of Higher State Educational Establishment of Ukraine «Ukrainian Medical Stomatological Academy», PhD in Medicine, Associate Professor This textbook is intended for undergraduate, postgraduate students and continuing education of health care professionals in a variety of clinical disciplines (medicine, pediatrics, dentistry) as it includes the basic concepts of human anatomy of the skull in adults and newborns. Rewiewed by: O.M. Slobodian, Head of the Department of Anatomy, Topographic Anatomy and Operative Surgery of Higher State Educational Establishment of Ukraine «Bukovinian State Medical University», Doctor of Medical Sciences, Professor M.V. -

Morphology of the Foramen Magnum in Young Eastern European Adults
Folia Morphol. Vol. 71, No. 4, pp. 205–216 Copyright © 2012 Via Medica O R I G I N A L A R T I C L E ISSN 0015–5659 www.fm.viamedica.pl Morphology of the foramen magnum in young Eastern European adults F. Burdan1, 2, J. Szumiło3, J. Walocha4, L. Klepacz5, B. Madej1, W. Dworzański1, R. Klepacz3, A. Dworzańska1, E. Czekajska-Chehab6, A. Drop6 1Department of Human Anatomy, Medical University of Lublin, Lublin, Poland 2St. John’s Cancer Centre, Lublin, Poland 3Department of Clinical Pathomorphology, Medical University of Lublin, Lublin, Poland 4Department of Anatomy, Collegium Medicum, Jagiellonian University, Krakow, Poland 5Department of Psychiatry and Behavioural Sciences, Behavioural Health Centre, New York Medical College, Valhalla NY, USA 6Department of General Radiology and Nuclear Medicine, Medical University of Lublin, Lublin, Poland [Received 21 July 2012; Accepted 7 September 2012] Background: The foramen magnum is an important anatomical opening in the base of the skull through which the posterior cranial fossa communicates with the vertebral canal. It is also related to a number of pathological condi- tions including Chiari malformations, various tumours, and occipital dysplasias. The aim of the study was to evaluate the morphology of the foramen magnum in adult individuals in relation to sex. Material and methods: The morphology of the foramen magnum was evalu- ated using 3D computer tomography images in 313 individuals (142 male, 171 female) aged 20–30 years. Results: The mean values of the foramen length (37.06 ± 3.07 vs. 35.47 ± ± 2.60 mm), breadth (32.98 ± 2.78 vs. 30.95 ± 2.71 mm) and area (877.40 ± ± 131.64 vs. -

Orangutan Positional Behavior and the Nature of Arboreal Locomotion in Hominoidea Susannah K.S
AMERICAN JOURNAL OF PHYSICAL ANTHROPOLOGY 000:000–000 (2006) Orangutan Positional Behavior and the Nature of Arboreal Locomotion in Hominoidea Susannah K.S. Thorpe1* and Robin H. Crompton2 1School of Biosciences, University of Birmingham, Edgbaston, Birmingham B15 2TT, UK 2Department of Human Anatomy and Cell Biology, University of Liverpool, Liverpool L69 3GE, UK KEY WORDS Pongo pygmaeus; posture; orthograde clamber; forelimb suspend ABSTRACT The Asian apes, more than any other, are and orthograde compressive locomotor modes are ob- restricted to an arboreal habitat. They are consequently an served more frequently. Given the complexity of orangu- important model in the interpretation of the morphological tan positional behavior demonstrated by this study, it is commonalities of the apes, which are locomotor features likely that differences in positional behavior between associated with arboreal living. This paper presents a de- studies reflect differences in the interplay between the tailed analysis of orangutan positional behavior for all age- complex array of variables, which were shown to influence sex categories and during a complete range of behavioral orangutan positional behavior (Thorpe and Crompton [2005] contexts, following standardized positional mode descrip- Am. J. Phys. Anthropol. 127:58–78). With the exception tions proposed by Hunt et al. ([1996] Primates 37:363–387). of pronograde suspensory posture and locomotion, orang- This paper shows that orangutan positional behavior is utan positional behavior is similar to that of the African highly complex, representing a diverse spectrum of posi- apes, and in particular, lowland gorillas. This study sug- tional modes. Overall, all orthograde and pronograde sus- gests that it is orthogrady in general, rather than fore- pensory postures are exhibited less frequently in the pres- limb suspend specifically, that characterizes the posi- ent study than previously reported. -
Lab Manual Axial Skeleton Atla
1 PRE-LAB EXERCISES When studying the skeletal system, the bones are often sorted into two broad categories: the axial skeleton and the appendicular skeleton. This lab focuses on the axial skeleton, which consists of the bones that form the axis of the body. The axial skeleton includes bones in the skull, vertebrae, and thoracic cage, as well as the auditory ossicles and hyoid bone. In addition to learning about all the bones of the axial skeleton, it is also important to identify some significant bone markings. Bone markings can have many shapes, including holes, round or sharp projections, and shallow or deep valleys, among others. These markings on the bones serve many purposes, including forming attachments to other bones or muscles and allowing passage of a blood vessel or nerve. It is helpful to understand the meanings of some of the more common bone marking terms. Before we get started, look up the definitions of these common bone marking terms: Canal: Condyle: Facet: Fissure: Foramen: (see Module 10.18 Foramina of Skull) Fossa: Margin: Process: Throughout this exercise, you will notice bold terms. This is meant to focus your attention on these important words. Make sure you pay attention to any bold words and know how to explain their definitions and/or where they are located. Use the following modules to guide your exploration of the axial skeleton. As you explore these bones in Visible Body’s app, also locate the bones and bone markings on any available charts, models, or specimens. You may also find it helpful to palpate bones on yourself or make drawings of the bones with the bone markings labeled. -

The Skull O Neurocranium, Form and Function O Dermatocranium, Form
Lesson 15 ◊ Lesson Outline: ♦ The Skull o Neurocranium, Form and Function o Dermatocranium, Form and Function o Splanchnocranium, Form and Function • Evolution and Design of Jaws • Fate of the Splanchnocranium ♦ Trends ◊ Objectives: At the end of this lesson, you should be able to: ♦ Describe the structure and function of the neurocranium ♦ Describe the structure and function of the dermatocranium ♦ Describe the origin of the splanchnocranium and discuss the various structures that have evolved from it. ♦ Describe the structure and function of the various structures that have been derived from the splanchnocranium ♦ Discuss various types of jaw suspension and the significance of the differences in each type ◊ References: ♦ Chapter: 9: 162-198 ◊ Reading for Next Lesson: ♦ Chapter: 9: 162-198 The Skull: From an anatomical perspective, the skull is composed of three parts based on the origins of the various components that make up the final product. These are the: Neurocranium (Chondocranium) Dermatocranium Splanchnocranium Each part is distinguished by its ontogenetic and phylogenetic origins although all three work together to produce the skull. The first two are considered part of the Cranial Skeleton. The latter is considered as a separate Visceral Skeleton in our textbook. Many other morphologists include the visceral skeleton as part of the cranial skeleton. This is a complex group of elements that are derived from the ancestral skeleton of the branchial arches and that ultimately gives rise to the jaws and the skeleton of the gill -

MORPHOMETRIC and MORPHOLOGICAL ANALYSIS of FORAMEN OVALE in DRY HUMAN SKULLS Ashwini
International Journal of Anatomy and Research, Int J Anat Res 2017, Vol 5(1):3547-51. ISSN 2321-4287 Original Research Article DOI: https://dx.doi.org/10.16965/ijar.2017.109 MORPHOMETRIC AND MORPHOLOGICAL ANALYSIS OF FORAMEN OVALE IN DRY HUMAN SKULLS Ashwini. N.S *1, Venkateshu. K.V 2. *1 Assistant Professor, Department Of Anatomy,Sri Devaraj Urs Medical College ,Tamaka, Kolar, Karnataka, India. 2 Professor And Head, Department Of Anatomy ,Sri Devaraj Urs Medical College, Tamaka, Kolar, India. ABSTRACT Introduction: Foramen ovale is an important foramen in the middle cranial fossa. Foramen ovale is situated in the greater wing of sphenoid bone, posterior to the foramen rotandum. Through the foramen ovale the mandibular nerve, accessory meningeal artery, lesser petrosal nerve, emissary veins pass .The shape of the foramen ovale is usually oval compared to other foramen of the skull. Materials and Methods: The study was conducted on 55 dry human skulls(110 sides) in Department of Anatomy, Sri Devaraj Urs Medical college, Tamaka, Kolar. Skulls in poor condition or skulls with partially damaged surroundings around the foramen ovale were excluded from the study. Linear measurements were taken on right and left sides of foramen ovale using divider and meter rule. Results: The maximum length of foramen ovale was 14 mm on the right side and 17 mm on the left, its maximum breadth on the right side was 8mm and 10mm on the left . Through the statistical analysis of morphometric measurements between right and left foramen ovale which was found to be insignificant , the results of both sides marks as the evidence of asymmetry in the morphometry of the foramen ovale. -

MBB: Head & Neck Anatomy
MBB: Head & Neck Anatomy Skull Osteology • This is a comprehensive guide of all the skull features you must know by the practical exam. • Many of these structures will be presented multiple times during upcoming labs. • This PowerPoint Handout is the resource you will use during lab when you have access to skulls. Mind, Brain & Behavior 2021 Osteology of the Skull Slide Title Slide Number Slide Title Slide Number Ethmoid Slide 3 Paranasal Sinuses Slide 19 Vomer, Nasal Bone, and Inferior Turbinate (Concha) Slide4 Paranasal Sinus Imaging Slide 20 Lacrimal and Palatine Bones Slide 5 Paranasal Sinus Imaging (Sagittal Section) Slide 21 Zygomatic Bone Slide 6 Skull Sutures Slide 22 Frontal Bone Slide 7 Foramen RevieW Slide 23 Mandible Slide 8 Skull Subdivisions Slide 24 Maxilla Slide 9 Sphenoid Bone Slide 10 Skull Subdivisions: Viscerocranium Slide 25 Temporal Bone Slide 11 Skull Subdivisions: Neurocranium Slide 26 Temporal Bone (Continued) Slide 12 Cranial Base: Cranial Fossae Slide 27 Temporal Bone (Middle Ear Cavity and Facial Canal) Slide 13 Skull Development: Intramembranous vs Endochondral Slide 28 Occipital Bone Slide 14 Ossification Structures/Spaces Formed by More Than One Bone Slide 15 Intramembranous Ossification: Fontanelles Slide 29 Structures/Apertures Formed by More Than One Bone Slide 16 Intramembranous Ossification: Craniosynostosis Slide 30 Nasal Septum Slide 17 Endochondral Ossification Slide 31 Infratemporal Fossa & Pterygopalatine Fossa Slide 18 Achondroplasia and Skull Growth Slide 32 Ethmoid • Cribriform plate/foramina -

Verhaegen M. the Aquatic Ape Evolves
HUMAN EVOLUTION Vol. 28 n.3-4 (237-266) - 2013 Verhaegen M. The Aquatic Ape Evolves: Common Miscon- Study Center for Anthropology, ceptions and Unproven Assumptions About Mechelbaan 338, 2580 Putte, the So-Called Aquatic Ape Hypothesis Belgium E-mail: [email protected] While some paleo-anthropologists remain skeptical, data from diverse biological and anthropological disciplines leave little doubt that human ancestors were at some point in our past semi- aquatic: wading, swimming and/or diving in shallow waters in search of waterside or aquatic foods. However, the exact sce- nario — how, where and when these semi-aquatic adaptations happened, how profound they were, and how they fit into the KEY WORDS: human evolution, hominid fossil record — is still disputed, even among anthro- Littoral theory, Aquarboreal pologists who assume some semi-aquatic adaptations. theory, aquatic ape, AAT, Here, I argue that the most intense phase(s) of semi-aquatic Archaic Homo, Homo erectus, adaptation in human ancestry occurred when populations be- Neanderthal, bipedalism, speech longing to the genus Homo adapted to slow and shallow littoral origins, Alister Hardy, Elaine diving for sessile foods such as shellfish during part(s) of the Morgan, comparative biology, Pleistocene epoch (Ice Ages), possibly along African or South- pachyosteosclerosis. Asian coasts. Introduction The term aquatic ape gives an incorrect impression of our semi-aquatic ancestors. Better terms are in my opinion the coastal dispersal model (Munro, 2010) or the littoral theory of human evolution, but although littoral seems to be a more appropriate biologi- cal term here than aquatic, throughout this paper I will use the well-known and common- ly used term AAH as shorthand for all sorts of waterside and semi-aquatic hypotheses. -

Topographical Anatomy and Morphometry of the Temporal Bone of the Macaque
Folia Morphol. Vol. 68, No. 1, pp. 13–22 Copyright © 2009 Via Medica O R I G I N A L A R T I C L E ISSN 0015–5659 www.fm.viamedica.pl Topographical anatomy and morphometry of the temporal bone of the macaque J. Wysocki 1Clinic of Otolaryngology and Rehabilitation, II Medical Faculty, Warsaw Medical University, Poland, Kajetany, Nadarzyn, Poland 2Laboratory of Clinical Anatomy of the Head and Neck, Institute of Physiology and Pathology of Hearing, Poland, Kajetany, Nadarzyn, Poland [Received 7 July 2008; Accepted 10 October 2008] Based on the dissections of 24 bones of 12 macaques (Macaca mulatta), a systematic anatomical description was made and measurements of the cho- sen size parameters of the temporal bone as well as the skull were taken. Although there is a small mastoid process, the general arrangement of the macaque’s temporal bone structures is very close to that which is observed in humans. The main differences are a different model of pneumatisation and the presence of subarcuate fossa, which possesses considerable dimensions. The main air space in the middle ear is the mesotympanum, but there are also additional air cells: the epitympanic recess containing the head of malleus and body of incus, the mastoid cavity, and several air spaces on the floor of the tympanic cavity. The vicinity of the carotid canal is also very well pneuma- tised and the walls of the canal are very thin. The semicircular canals are relatively small, very regular in shape, and characterized by almost the same dimensions. The bony walls of the labyrinth are relatively thin. -

The Condylar Canal and Emissary Vein—A Comprehensive and Pictorial Review of Its Anatomy and Variation
Child's Nervous System (2019) 35:747–751 https://doi.org/10.1007/s00381-019-04120-4 REVIEW ARTICLE The condylar canal and emissary vein—a comprehensive and pictorial review of its anatomy and variation Stefan Lachkar1 & Shogo Kikuta1 & Joe Iwanaga1,2 & R. Shane Tubbs1,3 Received: 6 March 2019 /Accepted: 8 March 2019 /Published online: 21 March 2019 # Springer-Verlag GmbH Germany, part of Springer Nature 2019 Abstract The condylar canal and its associated emissary vein serve as vital landmarks during surgical interventions involving skull base surgery. The condylar canal serves to function as a bridge of communication from the intracranial to extracranial space. Variations of the condylar canal are extremely prevalent and can present as either bilateral, unilateral, or completely absent. Anatomical variations of the condylar canal pose as a potential risk to surgeons and radiologist during diagnosis as it could be misinterpreted for a glomus jugular tumor and require surgical intervention when one is not needed. Few literature reviews have articulated the condylar canal and its associated emissary vein through extensive imaging. This present paper aims to further the knowledge of anatomical variations and surgical anatomy involving the condylar canal through high-quality computed tomography (CT) images with cadaveric and dry bone specimens that have been injected with latex to highlight emissary veins arising from the condylar canal. Keywords Posterior condylar canal . Anatomical variation . Anatomy . Cadaver . Skull . Emissary vein Introduction the posterior cranial fossa near or in the jugular fossa (Figs. 3 and 4)[2, 7, 9]. Its contents include the condylar emissary The condylar canal serves as a vital passageway for venous vein, which connects the sigmoid sinus or superior jugular circulation (condylar emissary vein) (Fig. -

Abnormality of the Foramen Spinosum Due to a Variation in the Trajectory of the Middle Meningeal Artery: a Case Report in Human
THIEME 73 Abnormality of the Foramen Spinosum due to a Variation in the Trajectory of the Middle Meningeal Artery: A Case Report in Human Joel Henrique Ellwanger1 Deivis de Campos1,2 1 Department of Biology and Pharmacy, Laboratory of Histology and Address for correspondence Deivis de Campos, PhD, Departamento Pathology, University of Santa Cruz do Sul – UNISC, Santa Cruz do Sul, de Biologia e Farmácia, Laboratório de Histologia e Patologia, RS, Brazil Universidade de Santa Cruz do Sul, Av. Independência, 2293, Santa 2 Institute of Basic Health Sciences, Post Graduate Program in Cruz do Sul, RS, Brazil (e-mail: [email protected]). Neurosciences, Federal University of Rio Grande do Sul - UFRGS, RS, Brazil J Neurol Surg Rep 2013;74:73–76. Abstract Originating from the maxillary artery, the middle meningeal artery (MMA) is predomi- nantly periosteal irrigating the bone and dura mater. It enters the floor of the middle cranial fossa through the foramen spinosum, travels laterally through a middle fossa bony ridge, and curves over the previous upper-greater wing of the sphenoid, where it in a variable point is divided into frontal and parietal branches. The complex sequence of the MMA development gives many opportunities for variant anatomy. In a Caucasian Keywords cadaver skull of an approximately 35-year-old individual belonging to the didactical ► middle meningeal collection of the Laboratory of Human Anatomy at the University of Santa Cruz do Sul, artery Brazil, it was noted that the right foramen spinosum has an abnormal shape. In this ► anatomic variation report, we discuss an abnormality of the foramen spinosum due to a variation in the ► foramen spinosum trajectory of the MMA. -

Fleagle and Lieberman 2015F.Pdf
15 Major Transformations in the Evolution of Primate Locomotion John G. Fleagle* and Daniel E. Lieberman† Introduction Compared to other mammalian orders, Primates use an extraordinary diversity of locomotor behaviors, which are made possible by a complementary diversity of musculoskeletal adaptations. Primate locomotor repertoires include various kinds of suspension, bipedalism, leaping, and quadrupedalism using multiple pronograde and orthograde postures and employing numerous gaits such as walking, trotting, galloping, and brachiation. In addition to using different locomotor modes, pri- mates regularly climb, leap, run, swing, and more in extremely diverse ways. As one might expect, the expansion of the field of primatology in the 1960s stimulated efforts to make sense of this diversity by classifying the locomotor behavior of living primates and identifying major evolutionary trends in primate locomotion. The most notable and enduring of these efforts were by the British physician and comparative anatomist John Napier (e.g., Napier 1963, 1967b; Napier and Napier 1967; Napier and Walker 1967). Napier’s seminal 1967 paper, “Evolutionary Aspects of Primate Locomotion,” drew on the work of earlier comparative anatomists such as LeGros Clark, Wood Jones, Straus, and Washburn. By synthesizing the anatomy and behavior of extant primates with the primate fossil record, Napier argued that * Department of Anatomical Sciences, Health Sciences Center, Stony Brook University † Department of Human Evolutionary Biology, Harvard University 257 You are reading copyrighted material published by University of Chicago Press. Unauthorized posting, copying, or distributing of this work except as permitted under U.S. copyright law is illegal and injures the author and publisher. fig. 15.1 Trends in the evolution of primate locomotion.