Morphometric Variability in Round Goby Neogobius Melanostomus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

National Report on the Implementation of the Ramsar Convention on Wetlands
NATIONAL REPORT ON THE IMPLEMENTATION OF THE RAMSAR CONVENTION ON WETLANDS National Reports to be submitted to the 12th Meeting of the Conference of the Contracting Parties, Uruguay, 2015 Please submit the completed National Report in Microsoft Word format (.doc, 97-2003), as an electronic file (not a printed copy) and preferably by e-mail, to Alexia Dufour, Regional Affairs Officer, Ramsar Secretariat ([email protected]) by 1 September 2014. National Report Format for Ramsar COP12, page 2 The structure of the COP12 National Report Format The COP12 National Report Format (NRF) is in four sections: Section 1 provides the institutional information about the Administrative Authority and National Focal Points for the national implementation of the Convention. Section 2 is a ‘free-text’ section in which the Party is invited to provide a summary of various aspects of national implementation progress and recommendations for the future. Section 3 provides the 66 implementation indicator questions, grouped under each Convention implementation strategy in the Strategic Plan 2009-2015, and with an optional ‘free-text’ section under each indicator question in which the Contracting Party may, if it wishes, add further information on national implementation of that activity. Section 4 is an optional annex to allow any Contracting Party that so wishes to provide additional information regarding any or all of its Wetlands of International Importance (Ramsar Sites). General guidance for completing and submitting the COP12 National Report Format IMPORTANT – PLEASE READ THIS GUIDANCE SECTION BEFORE STARTING TO COMPLETE THE NATIONAL REPORT FORMAT 1. All Sections of the COP12 NRF should be completed in one of the Convention’s official languages (English, French, Spanish). -
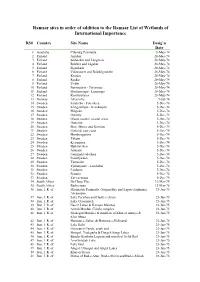
Ramsar Sites in Order of Addition to the Ramsar List of Wetlands of International Importance
Ramsar sites in order of addition to the Ramsar List of Wetlands of International Importance RS# Country Site Name Desig’n Date 1 Australia Cobourg Peninsula 8-May-74 2 Finland Aspskär 28-May-74 3 Finland Söderskär and Långören 28-May-74 4 Finland Björkör and Lågskär 28-May-74 5 Finland Signilskär 28-May-74 6 Finland Valassaaret and Björkögrunden 28-May-74 7 Finland Krunnit 28-May-74 8 Finland Ruskis 28-May-74 9 Finland Viikki 28-May-74 10 Finland Suomujärvi - Patvinsuo 28-May-74 11 Finland Martimoaapa - Lumiaapa 28-May-74 12 Finland Koitilaiskaira 28-May-74 13 Norway Åkersvika 9-Jul-74 14 Sweden Falsterbo - Foteviken 5-Dec-74 15 Sweden Klingavälsån - Krankesjön 5-Dec-74 16 Sweden Helgeån 5-Dec-74 17 Sweden Ottenby 5-Dec-74 18 Sweden Öland, eastern coastal areas 5-Dec-74 19 Sweden Getterön 5-Dec-74 20 Sweden Store Mosse and Kävsjön 5-Dec-74 21 Sweden Gotland, east coast 5-Dec-74 22 Sweden Hornborgasjön 5-Dec-74 23 Sweden Tåkern 5-Dec-74 24 Sweden Kvismaren 5-Dec-74 25 Sweden Hjälstaviken 5-Dec-74 26 Sweden Ånnsjön 5-Dec-74 27 Sweden Gammelstadsviken 5-Dec-74 28 Sweden Persöfjärden 5-Dec-74 29 Sweden Tärnasjön 5-Dec-74 30 Sweden Tjålmejaure - Laisdalen 5-Dec-74 31 Sweden Laidaure 5-Dec-74 32 Sweden Sjaunja 5-Dec-74 33 Sweden Tavvavuoma 5-Dec-74 34 South Africa De Hoop Vlei 12-Mar-75 35 South Africa Barberspan 12-Mar-75 36 Iran, I. R. -

THE REVIEW of ECOLOGICAL and GENETIC RESEARCH of PONTO-CASPIAN GOBIES (Pisces, Gobiidae) in EUROPE
Croatian Journal of Fisheries, 2016, 74, 110-123 G. Jakšić et al: Ecological and genetic research of Ponto-Caspian gobies DOI: 10.1515/cjf-2016-0015 CODEN RIBAEG ISSN 1330-061X (print), 1848-0586 (online) THE REVIEW OF ECOLOGICAL AND GENETIC RESEARCH OF PONTO-CASPIAN GOBIES (Pisces, Gobiidae) IN EUROPE Goran Jakšić1, *, Margita Jadan2, Marina Piria3 1City of Karlovac, Banjavčićeva 9, 47000 Karlovac, Croatia 2Division of materials chemistry, Ruđer Bošković Institute, Bijenička 54, 10000 Zagreb, Croatia 3University of Zagreb, Faculty of Agriculture, Department of Fisheries, Beekeeping, Game management and Special Zoology, Svetošimunska 25, 10000 Zagreb, Croatia *Corresponding Author, Email: [email protected] ARTICLE INFO ABSTRACT Received: 27 January 2016 Invasive Ponto-Caspian gobies (monkey goby Neogobius fluviatilis, round Received in revised form: 14 May 2016 goby Neogobius melanostomus and bighead goby Ponticola kessleri) have Accepted: 20 May 2016 recently caused dramatic changes in fish assemblage structure throughout Available online: 24 May 2016 European river systems. This review provides summary of recent research on their dietary habits, age and growth, phylogenetic lineages and gene diversity. The principal food of all three species is invertebrates, and more rarely fish, which depends on the type of habitat, part of the year, as well as the morphological characteristics of species. According to the von Bertalanffy growth model, size at age is specific for the region, but due to its disadvantages it is necessary to test other growth models. Phylogenetic Keywords: analysis of monkey goby and round goby indicates separation between the European river systems Black Sea and the Caspian Sea haplotypes. The greatest genetic diversity is Invasive gobies found among populations of the Black Sea, and the lowest among European Ecology invaders. -

Slender-Billed Curlew: Promising Discovery in the Danube Delta 51 Slender-Billed Curlew: Promising Discovery in the Danube Delta
Zhmud: Slender-billed Curlew: promising discovery in the Danube delta 51 Slender-billed Curlew: promising discovery in the Danube delta MYKHAYLO ZHMUD Danube Delta Biosphere Reserve, T. Vosstaniya Str. 132a, Vilkovo Odessa Reg. Ukraine UA68355. [email protected] Zhmud, M. 2005. Slender-billed Curlew: promising discovery in the Danube delta. Wader Study Group Bull. 106: 51–54. Keywords: shorebird, Slender-billed Curlew, Numenius tenuirostris, endangered species, Danube delta, Ukraine. Despite the hard work of the world conservation community to study the Slender-billed Curlew Numenius tenuirostris, discover its nesting sites and bring it back from the brink of extinction, it still remains a mystery bird. Currently, it is one of the rarest birds of the Old World and is critically endangered. Therefore, any information about recent records is of vital importance. Here, I report on the status of the species at one of its most important migrating stopover sites, the Danube delta, where promising observations were made in 2003 and 2004. As a consequence, plans are being made for follow-up studies and conservation activities. INTRODUCTION OBSERVATIONS OF SLENDER-BILLED CURLEWS DURING 2003–2004 The Danube delta, as well as the whole of the N and NW Black Sea coast is located on the main Slender-billed Cur- On 25 Jul 2003, I saw four Slender-billed Curlews together on lew migration route between its supposed W Siberian nest- the north-west end of the Taranova spit (45.443°N, 29.775°E) ing sites and its supposed Mediterranean wintering sites in the northern part of the delta between the channels Prorva (Gretton 1991; Heredia et al. -

Chapter 5 Drainage Basin of the Black Sea
165 CHAPTER 5 DRAINAGE BASIN OF THE BLACK SEA This chapter deals with the assessment of transboundary rivers, lakes and groundwa- ters, as well as selected Ramsar Sites and other wetlands of transboundary importance, which are located in the basin of the Black Sea. Assessed transboundary waters in the drainage basin of the Black Sea Transboundary groundwaters Ramsar Sites/wetlands of Basin/sub-basin(s) Recipient Riparian countries Lakes in the basin within the basin transboundary importance Rezovska/Multudere Black Sea BG, TR Danube Black Sea AT, BA, BG, Reservoirs Silurian-Cretaceous (MD, RO, Lower Danube Green Corridor and HR, CZ, DE, Iron Gate I and UA), Q,N1-2,Pg2-3,Cr2 (RO, UA), Delta Wetlands (BG, MD, RO, UA) HU, MD, ME, Iron Gate II, Dobrudja/Dobrogea Neogene- RO, RS, SI, Lake Neusiedl Sarmatian (BG-RO), Dobrudja/ CH, UA Dobrogea Upper Jurassic-Lower Cretaceous (BG-RO), South Western Backa/Dunav aquifer (RS, HR), Northeast Backa/ Danube -Tisza Interfluve or Backa/Danube-Tisza Interfluve aquifer (RS, HU), Podunajska Basin, Zitny Ostrov/Szigetköz, Hanság-Rábca (HU), Komarnanska Vysoka Kryha/Dunántúli – középhegység északi rész (HU) - Lech Danube AT, DE - Inn Danube AT, DE, IT, CH - Morava Danube AT, CZ, SK Floodplains of the Morava- Dyje-Danube Confluence --Dyje Morava AT, CZ - Raab/Rába Danube AT, HU Rába shallow aquifer, Rába porous cold and thermal aquifer, Rába Kőszeg mountain fractured aquifer, Günser Gebirge Umland, Günstal, Hügelland Raab Ost, Hügelland Raab West, Hügelland Rabnitz, Lafnitztal, Pinkatal 1, Pinkatal 2, Raabtal, -

Population Dynamics and Feeding Ecology of the Invasive Caucasian Dwarf Goby, Knipowitschia Caucasica, in a Freshwater Habitat in Ukraine
Knowl. Manag. Aquat. Ecosyst. 2020, 421, 26 Knowledge & © A. Didenko et al., Published by EDP Sciences 2020 Management of Aquatic https://doi.org/10.1051/kmae/2020018 Ecosystems Journal fully supported by Office www.kmae-journal.org français de la biodiversité RESEARCH PAPER Population dynamics and feeding ecology of the invasive Caucasian dwarf goby, Knipowitschia caucasica, in a freshwater habitat in Ukraine Alexander Didenko1,*, Igor Buzevych1, Yuriy Volikov2, Svitlana Kruzhylina1 and Alexander Gurbyk1 1 Institute of Fisheries of the National Academy of Agrarian Sciences of Ukraine, Obukhivska St. 135, 03164 Kyiv, Ukraine 2 Institute of Hydrobiology of the National Academy of Sciences of Ukraine, Prospekt Geroiv Stalingradu, 12, 04210 Kyiv, Ukraine Received: 25 March 2020 / Accepted: 9 May 2020 Abstract – Population dynamics and feeding patterns of invasive Knipowitschia caucasica were studied in the littoral zone of the lower Stugna River. The abundances of this goby showed significant inter-annual and seasonal fluctuations. The studied population of K. caucasica was represented by two age groups (0 and I). Fish die after their first breeding season. In total, 58 prey items were identified in the diet of K. caucasica at the sampling site. The most abundant prey were copepods and cladocerans, while the most frequently encountered prey were copepods and chironomid larvae. Copepods were represented mainly by Cyclopoidae. Cladocerans included 21 taxa, among which the most abundant were Diaphanosoma sp., Acroperus harpae, and Disparalona rostrata; chironomids included 22 taxa, among which the most abundant was Cricotopus sylvestris. The diet composition showed seasonal dynamics, where copepods predominated in January to April and in August-September, chironomid larvae were especially important in May to July, while cladocerans were most important in November-December. -

Important Bird Areas and Potential Ramsar Sites in Europe
cover def. 25-09-2001 14:23 Pagina 1 BirdLife in Europe In Europe, the BirdLife International Partnership works in more than 40 countries. Important Bird Areas ALBANIA and potential Ramsar Sites ANDORRA AUSTRIA BELARUS in Europe BELGIUM BULGARIA CROATIA CZECH REPUBLIC DENMARK ESTONIA FAROE ISLANDS FINLAND FRANCE GERMANY GIBRALTAR GREECE HUNGARY ICELAND IRELAND ISRAEL ITALY LATVIA LIECHTENSTEIN LITHUANIA LUXEMBOURG MACEDONIA MALTA NETHERLANDS NORWAY POLAND PORTUGAL ROMANIA RUSSIA SLOVAKIA SLOVENIA SPAIN SWEDEN SWITZERLAND TURKEY UKRAINE UK The European IBA Programme is coordinated by the European Division of BirdLife International. For further information please contact: BirdLife International, Droevendaalsesteeg 3a, PO Box 127, 6700 AC Wageningen, The Netherlands Telephone: +31 317 47 88 31, Fax: +31 317 47 88 44, Email: [email protected], Internet: www.birdlife.org.uk This report has been produced with the support of: Printed on environmentally friendly paper What is BirdLife International? BirdLife International is a Partnership of non-governmental conservation organisations with a special focus on birds. The BirdLife Partnership works together on shared priorities, policies and programmes of conservation action, exchanging skills, achievements and information, and so growing in ability, authority and influence. Each Partner represents a unique geographic area or territory (most often a country). In addition to Partners, BirdLife has Representatives and a flexible system of Working Groups (including some bird Specialist Groups shared with Wetlands International and/or the Species Survival Commission (SSC) of the World Conservation Union (IUCN)), each with specific roles and responsibilities. I What is the purpose of BirdLife International? – Mission Statement The BirdLife International Partnership strives to conserve birds, their habitats and global biodiversity, working with people towards sustainability in the use of natural resources. -

SOUTHERN UKRAINE Settler
© Lonely Planet Publications 160 lonelyplanet.com SOUTHERN UKRAINE •• Odesa 161 Southern Ukraine ODESA ОДЕСА Local writer Issac Babel claimed Odesa %048 (7-digit Nos), 0482 (6-digit Nos) / pop one had ‘more charm than any city in the Russian million Empire’ and that’s probably still true in Південна Odesa is a city straight from literature – an modern-day Ukraine. The source of this energetic, decadent boomtown. Its famous charm is Odesans themselves: a breed apart, Potemkin Steps sweep down to the Black they’re stylish, cultured, funny, savvy and not Україна Sea and Ukraine’s biggest commercial port. easily impressed. Behind them, a cosmopolitan cast of char- This southerly region once helped make the Russian empress Catherine great. As part of her acters makes merry among pastel neoclas- History territorial acquisitions in the late 18th century, it vastly expanded her dominion and brought sical buildings lining a geometrical grid of Catherine the Great imagined Odesa as the St Russia huge wealth by opening it up to the Black Sea. Novorossiya, or new Russia, as it was leafy streets. Petersburg of the South. Her lover, General ambitiously christened, had been a Wild West–style no-man’s-land between the domain of Immigrants from all over Europe were Grygory Potemkin, laid the groundwork for invited to make their fortune here when her dream in 1789 by capturing the Turkish the Cossacks and that of the Crimean Tatars. Under Catherine it became a melting pot, as Odesa was founded in the late 18th century fortress of Hadjibey, which previously stood Bulgarians, Germans, Greeks, Italians, Moldavians, Russians, Swedes and many others were by Russia’s Catherine the Great. -

Southern Ukraine Південна Україна
160 Southern Ukraine Південна Україна This southerly region once helped make the Russian empress Catherine great. As part of her territorial acquisitions in the late 18th century, it vastly expanded her dominion and brought Russia huge wealth by opening it up to the Black Sea. Novorossiya, or new Russia, as it was ambitiously christened, had been a Wild West–style no-man’s-land between the domain of the Cossacks and that of the Crimean Tatars. Under Catherine it became a melting pot, as Bulgarians, Germans, Greeks, Italians, Moldavians, Russians, Swedes and many others were invited to populate the area and set up business along the coast to trade. This history, coupled with a temperate climate, has shaped the character of the region today, especially the largest city, Odesa (Odessa in Russian). Entrepreneurial and cosmopolitan, Odesa is also Ukraine’s capital of hedonism. Closer than Crimea to Kyiv, with sandy beaches and a wicked nightlife, it’s a favourite weekend break from the capital and is, in many ways, cooler. Southern Ukraine has, however, more to offer than just Odesa’s joie de vivre and attitude: it’s also home to three major river estuaries. The Dnipro empties into the Black Sea 60km east of Odesa; the Dnister, 40km southwest. But the most spectacular estuary – and, sadly, the most threatened – is that of the Danube in the country’s far southwest corner. Here, in the small Ukrainian nook of Europe’s largest wetlands, you’ll find more than 300 different bird species and animals such as mink, freshwater otters and monk seals. -

Download This Article in PDF Format
Knowl. Manag. Aquat. Ecosyst. 2020, 421, 9 Knowledge & © Z. Csabai et al., Published by EDP Sciences 2020 Management of Aquatic https://doi.org/10.1051/kmae/2020003 Ecosystems Journal fully supported by Office www.kmae-journal.org français de la biodiversité RESEARCH PAPER Mass appearance of the Ponto-Caspian invader Pontogammarus robustoides in the River Tisza catchment: bypass in the southern invasion corridor? Zoltán Csabai1,*, Péter Borza2, Tomasz Rewicz3,4, Bálint Pernecker1, Balázs J. Berta1 and Arnold Móra1 1 University of Pécs, Faculty of Sciences, Department of Hydrobiology, Ifjuság utja 6, 7624 Pécs, Hungary 2 MTA Centre for Ecological Research, Danube Research Institute, Karolina ut 29, 1113 Budapest, Hungary 3 University of Lodz, Department of Invertebrate Zoology & Hydrobiology, Banacha 12/16, 90-237 Lodz, Poland 4 University of Guelph, Centre for Biodiversity Genomics, 50 Stone Road East, Guelph, ON, N1G2W1, Canada Received: 25 November 2019 / Accepted: 14 January 2020 Abstract – The river Danube is the backbone of the ‘southern invasion corridor’, one of the most important passages for the spread of Ponto-Caspian invaders in Europe. However, not all of these species used the passive or active upstream movement in the main channel to reach the upper sections and tributaries, some found detours. Mass occurrences of the Ponto-Caspian peracarid, Pontogammarus robustoides (Sars, 1894) were recorded at 17 sites along the entire Hungarian section of the River Maros, for the first time in the River Tisza catchment and also in Hungary. Those populations are found ca. 707 km upstream from the closest known and confirmed locality in the lower Danube section. -

Ukraine, Danube Delta Biodiversitly
GLOBAL ENVIRONMENT FACILITY Public Disclosure Authorized - - Ukraine,; DanubeDelta Biodiversitly * \ - N - * > 4 j;, * X~~~- * ~ ~ .. .. Public Disclosure Authorized Public Disclosure Authorized Proje'ctDocument / _ _ My1_994 _ , _ !- 4 , _ t ts-- Public Disclosure Authorized THEWORLD 9SANK GEF Documentation fhe Global Environment Facility (GEF) assistsdeveloping countries to protect the global environmentin four areas:global warming,pollution of internationalwaters, destructionof biodiversity,.anddepletion of the ozonelayer. The GEF is jointly implemented -bytheUnited Nations Development Programme,the United Nations Environment Programme, and the World Bank. GEF Project Documents - identifiedby a green band- provideextended project- specificinformation. The implementing agency responsible for eachproject is identifiedby its logo on the coverof the document. GlobalEnvironment CoordinationDivision EnvironmentDepartment. World Bank 1818 H Street,NW Washington,DC 204h3 Telephone:(202) 473-1816 Fax:(202) 522-3256 Document of The World Bank FOR OFFICIAL USE ONLY Report No. 12575-UA GLOBAL ENVIRONMENT FACILITY MEMORANDUMAND RECOMMENDATION OF THE DIRECTOR EUROPE AND CENTRAL ASIA COUNTRY DEPARTMENTI OF THE INTERNATIONALBANK FOR RECONSTRUCTIONAND DEVELOPMENT TO THE REGIONALVICE PRESIDENT ON A GRANT FROM THE GLOBAL ENVIRONMENTTRUST FUND IN AN AMOUNT EQUIVALENTTO SDR 1.10 MILLION (US$1.50 MILLION) TO UKRAINE FOR A DANUBE DELTA BIODIVERSITY PROJECT PART I: MEMORANDUM AND RECOMMENDATION May 25, 1994 NaturalResource Management Division CountryDepartment -

Karadag Nature Reserve of National Academy of Science of Ukraine
Interpretative Trails on the Ground: Support to the Management of Natural Protected Areas in the Black Sea Region “InterTrails” GUIDELINES ON NATURE INTERPRETATION Odessa-2013 GUIDELINES ON NATURE INTERPRETATION Edited by Oleg Rubel, Anastasiya Snigirova Authors: Natalia Fedoronchuk, Vladimir Poltorak, Galina Bezvushko, Oleh Derkach, Tatyana Balatskaya, Mykola Rozhenko, Julia Nazarchuk This book has been prepared in the frame of the Project “Interpretative Trails on the Ground: Support to the Management of Natural Protected Areas in the Black Sea Region” (InterTrails) funded by European Union. The authors have developed methodological and methodical basis of interpretation of nature protected areas. The structure and detailed description of four ecological trails located in protected areas of the Black Sea coast: Karadag Natural Reserve of NAS of Ukraine, Danube Biosphere Reserve of NAS of Ukraine, Lower Dniester National Nature Park, Regional Landscape Park "Kinburn Sand-spit". The book is designed for tour guides, travel managers and campaigns, staff of protected area, researchers, students and wide range of readers. Reviewers: Alexander Voloshkevich, PhD, director of Danube Biosphere Reserve of National Academy of Science of Ukraine Natalia Gorіap, director of touristic company "Salіx" Michael Zhmud, Ph.D., director of the touristic company "Pelican-Vilkovo-Tour" Ivan Rusev, Ph.D., Ukrainian Anti-Plague Research Institute named after I. I. Mechnikov Vasiliy Fedorenko, PhD, deputy director of the Danube Biosphere Reserve of National