Uncorrected Proofs
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

National Report on the Implementation of the Ramsar Convention on Wetlands
NATIONAL REPORT ON THE IMPLEMENTATION OF THE RAMSAR CONVENTION ON WETLANDS National Reports to be submitted to the 12th Meeting of the Conference of the Contracting Parties, Uruguay, 2015 Please submit the completed National Report in Microsoft Word format (.doc, 97-2003), as an electronic file (not a printed copy) and preferably by e-mail, to Alexia Dufour, Regional Affairs Officer, Ramsar Secretariat ([email protected]) by 1 September 2014. National Report Format for Ramsar COP12, page 2 The structure of the COP12 National Report Format The COP12 National Report Format (NRF) is in four sections: Section 1 provides the institutional information about the Administrative Authority and National Focal Points for the national implementation of the Convention. Section 2 is a ‘free-text’ section in which the Party is invited to provide a summary of various aspects of national implementation progress and recommendations for the future. Section 3 provides the 66 implementation indicator questions, grouped under each Convention implementation strategy in the Strategic Plan 2009-2015, and with an optional ‘free-text’ section under each indicator question in which the Contracting Party may, if it wishes, add further information on national implementation of that activity. Section 4 is an optional annex to allow any Contracting Party that so wishes to provide additional information regarding any or all of its Wetlands of International Importance (Ramsar Sites). General guidance for completing and submitting the COP12 National Report Format IMPORTANT – PLEASE READ THIS GUIDANCE SECTION BEFORE STARTING TO COMPLETE THE NATIONAL REPORT FORMAT 1. All Sections of the COP12 NRF should be completed in one of the Convention’s official languages (English, French, Spanish). -
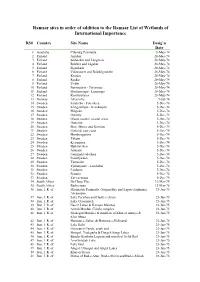
Ramsar Sites in Order of Addition to the Ramsar List of Wetlands of International Importance
Ramsar sites in order of addition to the Ramsar List of Wetlands of International Importance RS# Country Site Name Desig’n Date 1 Australia Cobourg Peninsula 8-May-74 2 Finland Aspskär 28-May-74 3 Finland Söderskär and Långören 28-May-74 4 Finland Björkör and Lågskär 28-May-74 5 Finland Signilskär 28-May-74 6 Finland Valassaaret and Björkögrunden 28-May-74 7 Finland Krunnit 28-May-74 8 Finland Ruskis 28-May-74 9 Finland Viikki 28-May-74 10 Finland Suomujärvi - Patvinsuo 28-May-74 11 Finland Martimoaapa - Lumiaapa 28-May-74 12 Finland Koitilaiskaira 28-May-74 13 Norway Åkersvika 9-Jul-74 14 Sweden Falsterbo - Foteviken 5-Dec-74 15 Sweden Klingavälsån - Krankesjön 5-Dec-74 16 Sweden Helgeån 5-Dec-74 17 Sweden Ottenby 5-Dec-74 18 Sweden Öland, eastern coastal areas 5-Dec-74 19 Sweden Getterön 5-Dec-74 20 Sweden Store Mosse and Kävsjön 5-Dec-74 21 Sweden Gotland, east coast 5-Dec-74 22 Sweden Hornborgasjön 5-Dec-74 23 Sweden Tåkern 5-Dec-74 24 Sweden Kvismaren 5-Dec-74 25 Sweden Hjälstaviken 5-Dec-74 26 Sweden Ånnsjön 5-Dec-74 27 Sweden Gammelstadsviken 5-Dec-74 28 Sweden Persöfjärden 5-Dec-74 29 Sweden Tärnasjön 5-Dec-74 30 Sweden Tjålmejaure - Laisdalen 5-Dec-74 31 Sweden Laidaure 5-Dec-74 32 Sweden Sjaunja 5-Dec-74 33 Sweden Tavvavuoma 5-Dec-74 34 South Africa De Hoop Vlei 12-Mar-75 35 South Africa Barberspan 12-Mar-75 36 Iran, I. R. -

Bird Numbers 2019
Bird Numbers 2019 Counting birds counts Book of Abstracts © Joaquim Antunes st 21 Conference of the European Bird Census Council ISBN: 978-989-8550-85-9 This page was intentionally left in blank Imprint Editors João E. Rabaça, Carlos Godinho, Inês Roque LabOr-Laboratory of Ornithology, ICAAM, University of Évora Scientific Committee Aleksi Lehikoinen (chair), Ruud Foppen, Lluís Brotons, Mark Eaton, Henning Heldbjerg, João E. Rabaça, Carlos Godinho, Rui Lourenço, Oskars Keišs, Verena Keller Organising Committee João E. Rabaça, Carlos Godinho, Inês Roque, Rui Lourenço, Pedro Pereira, Ruud Foppen, Aleksi Lehikoinen Volunteer team André Oliveira, Cláudia Lopes, Inês Guise, Patrícia Santos, Pedro Freitas, Pedro Ribeiro, Rui Silva, Sara Ornelas, Shirley van der Horst Recommended citation Rabaça, J.E., Roque, I., Lourenço, R. & Godinho, C. (Eds.) 2019: Bird Numbers 2019: counting birds counts. Book of Abstracts of the 21st Conference of the European Bird Census Council. University of Évora, Évora. ISBN: 978-989-8550-85-9 Bird Numbers 2019: counting birds counts The logo of the Conference pictures two species with different stories: the Woodchat Shrike Lanius senator and the Dartford Warbler Sylvia undata, both occurring in Alentejo. The first is a LC species currently suffering a moderate decline in Spain and Portugal; the second is a resident bird classified as NT which is declining in Europe at a moderate rate and seemingly increasing in Portugal, a country that holds 25% of its European population. Bird Numbers 2019 Counting birds counts -

Romania: Danube Delta Integrated Sustainable Development Strategy
Romania: Danube Delta Integrated Sustainable Development Strategy About the Danube Delta Region The Danube Delta is one of the continent’s most valuable habitats for specific delta wildlife and biodiversity. Established as a UNESCO Biosphere Reserve and a Ramsar site in 1990, it is the Europe’s second largest delta, and the best preserved of European deltas. The most significant physical and ecological feature of the Danube Delta is its vast expanse of wetlands, including freshwater marsh, lakes and ponds, streams and channels. With an area of 3,446 km2, is the world’s largest wetland. Only 9% of the area is permanently above water. The Delta hosts extraordinary biodiversity and provides important environmental services. It is the home of over 1,200 varieties of plants, 300 species of birds, as well as 45 freshwater fish species in its numerous lakes and marshes. There are 16 strictly protected areas in the delta where no economic activities are allowed, and areas for ecological rehabilitation and buffer zones between economical areas where tourist activities are permitted as long as the environment is protected. Dual Challenge in Developing the Danube Delta A dual challenge for the sustainable development of the Danube Delta is the conservation of its ecological assets and improvement of the quality of life for its residents. The Danube Delta is the largest remaining natural delta in Europe and one of the largest in the world. It is also the only river that is entirely contained within a Biosphere Reserve. It is important to conserve all of its ecological assets. 1 Danube Delta is perhaps one of the least inhabited regions of temperate Europe, with only about 10,000 people in one town (Sulina) and about 20 scattered villages. -

THE REVIEW of ECOLOGICAL and GENETIC RESEARCH of PONTO-CASPIAN GOBIES (Pisces, Gobiidae) in EUROPE
Croatian Journal of Fisheries, 2016, 74, 110-123 G. Jakšić et al: Ecological and genetic research of Ponto-Caspian gobies DOI: 10.1515/cjf-2016-0015 CODEN RIBAEG ISSN 1330-061X (print), 1848-0586 (online) THE REVIEW OF ECOLOGICAL AND GENETIC RESEARCH OF PONTO-CASPIAN GOBIES (Pisces, Gobiidae) IN EUROPE Goran Jakšić1, *, Margita Jadan2, Marina Piria3 1City of Karlovac, Banjavčićeva 9, 47000 Karlovac, Croatia 2Division of materials chemistry, Ruđer Bošković Institute, Bijenička 54, 10000 Zagreb, Croatia 3University of Zagreb, Faculty of Agriculture, Department of Fisheries, Beekeeping, Game management and Special Zoology, Svetošimunska 25, 10000 Zagreb, Croatia *Corresponding Author, Email: [email protected] ARTICLE INFO ABSTRACT Received: 27 January 2016 Invasive Ponto-Caspian gobies (monkey goby Neogobius fluviatilis, round Received in revised form: 14 May 2016 goby Neogobius melanostomus and bighead goby Ponticola kessleri) have Accepted: 20 May 2016 recently caused dramatic changes in fish assemblage structure throughout Available online: 24 May 2016 European river systems. This review provides summary of recent research on their dietary habits, age and growth, phylogenetic lineages and gene diversity. The principal food of all three species is invertebrates, and more rarely fish, which depends on the type of habitat, part of the year, as well as the morphological characteristics of species. According to the von Bertalanffy growth model, size at age is specific for the region, but due to its disadvantages it is necessary to test other growth models. Phylogenetic Keywords: analysis of monkey goby and round goby indicates separation between the European river systems Black Sea and the Caspian Sea haplotypes. The greatest genetic diversity is Invasive gobies found among populations of the Black Sea, and the lowest among European Ecology invaders. -

Evaluation of Wetlands and Floodplain Areas in the Danube River Basin Final Report May 1999
DANUBE POLLUTION REDUCTION PROGRAMME EVALUATION OF WETLANDS AND FLOODPLAIN AREAS IN THE DANUBE RIVER BASIN FINAL REPORT MAY 1999 Programme Coordination Unit UNDP/GEF Assistance prepared by WWF Danube-Carpathian-Programme and WWF-Auen-Institut (Germany) DANUBE POLLUTION REDUCTION PROGRAMME EVALUATION OF WETLANDS AND FLOODPLAIN AREAS IN THE DANUBE RIVER BASIN FINAL REPORT MAY 1999 Programme Coordination Unit UNDP/GEF Assistance prepared by WWF Danube-Carpathian-Programme and WWF-Auen-Institut (Germany) Preface The "Evaluation of Wetlands and Flkoodplain Areas in the Danube River Basin" study was prepared in the frame of the Danube Pollution Reduction Programme (PRP). The Study has been undertaken to define priority wetland and floodplain rehabilitation sites as a component of the Pollution reduction Programme. The present report addresses the identification of former floodplains and wetlands in the Danube River Basin, as well as the description of the current status and evaluation of the ecological importance of the potential for rehabilitation. Based on this evaluation, 17 wetland/floodplain sites have been identified for rehabilitation considering their ecological importance, their nutrient removal capacity and their role in flood protection. Most of the identified wetlands will require transboundary cooperation and represent an important first step in retoring the ecological balance in the Danube River Basin. The results are presented in the form of thematic maps that can be found in Annex I of the study. The study was prepared by the WWF-Danube-Carpathian-Programme and the WWF-Auen-Institut (Institute for Floodplains Ecology, WWF-Germany), under the guidance of the UNDP/GEF team of experts of the Danube Programme Coordination Unit (DPCU) in Vienna, Austria. -

Sedimentary Microplastic Concentrations from the Romanian
www.nature.com/scientificreports OPEN Sedimentary microplastic concentrations from the Romanian Danube River to the Black Sea Iulian Pojar1, Adrian Stănică1*, Friederike Stock2, Christian Kochleus2, Michael Schultz1 & Chris Bradley3 A multitude of recent studies have detailed microplastic concentrations in aquatic and terrestrial environments, although questions remain over their ultimate fate. At present, few studies have detailed microplastic characteristics and abundance along a freshwater–marine interface, and considerable uncertainties remain over the modelled contribution of terrestrial and riverine microplastic to the world’s oceans. In this article, for the frst time, we detail sedimentary microplastic concentrations along a River–Sea transect from the lower reaches of a major continental river, the River Danube, through the Danube Delta, the Black Sea coast to the Romanian and Bulgarian inner shelf of the Black Sea. Our results indicate that isolated areas of the Danube Delta are still relatively pristine, with few microplastic particles in some of the sediments sampled. A multitude of recent studies have detailed microplastic concentrations in aquatic and terrestrial environments, although questions remain over their ultimate fate. At present, few studies have detailed microplastic charac- teristics and abundance along a freshwater–marine interface, and considerable uncertainties remain over the modelled contribution of terrestrial and riverine microplastic to the world’s oceans. In this article, for the frst time, we detail sedimentary microplastic concentrations along a River–Sea transect from the lower reaches of a major continental river, the River Danube, through the Danube Delta, the Black Sea coast to the Romanian and Bulgarian inner shelf of the Black Sea. Our results indicate that isolated areas of the Danube Delta are still relatively pristine, with few microplastic particles in some of the sediments sampled. -

National Report Bulgaria
NATIONAL REPORT BULGARIA AGREEMENT ON THE CONSERVATION OF AFRICAN-EURASIAN MIGRATORY WATERBIRDS (The Hague, 1995) Implementation during the period 2006 and 2008 Contracting Party: BULGARIA Designated AEWA Administrative Authority: Ministry of Environment and Water Full name of the institution: Name and title of the head of the institution: Dzhevdet Chakarov - Minister Mailing address: 22, Maria Luisa Blvd Telephone: (+ 359 2) 988 25 77 Fax: (+ 359 2) 986 25 33 Email: [email protected] Name and title (if different) of the designated contact officer for AEWA matters: Valeri Georgiev Mailing address (if different) for the designated contact officer: 22, Maria Luisa Blvd Telephone: (+ 359 2) 940 6151 Fax: (+ 359 2) 940 6127 Email: [email protected] 2 Table of Contents 1. Overview of Action Plan implementation 5 2. Species conservation 6 Legal measures 6 Single Species Action Plans 8 Emergency measures 8 Re-establishments 8 Introductions 8 3. Habitat conservation 8 Habitat inventories 8 Conservation of areas 9 Rehabilitation and restoration 10 4. Management of human activities 11 Hunting 13 Eco-tourism 14 Other human activities 14 5. Research and monitoring 15 Status of research and monitoring programmes for species 15 6. Education and information 16 Training and development programmes 16 Raising public awareness 16 7. Final comments 17 8. Progress to implement Resolutions and Recommendations of the Meeting of the Parties 17 9. OPTIONAL SECTION – Planned and future actions 17 List of abbreviations and acronyms used in the -

Slender-Billed Curlew: Promising Discovery in the Danube Delta 51 Slender-Billed Curlew: Promising Discovery in the Danube Delta
Zhmud: Slender-billed Curlew: promising discovery in the Danube delta 51 Slender-billed Curlew: promising discovery in the Danube delta MYKHAYLO ZHMUD Danube Delta Biosphere Reserve, T. Vosstaniya Str. 132a, Vilkovo Odessa Reg. Ukraine UA68355. [email protected] Zhmud, M. 2005. Slender-billed Curlew: promising discovery in the Danube delta. Wader Study Group Bull. 106: 51–54. Keywords: shorebird, Slender-billed Curlew, Numenius tenuirostris, endangered species, Danube delta, Ukraine. Despite the hard work of the world conservation community to study the Slender-billed Curlew Numenius tenuirostris, discover its nesting sites and bring it back from the brink of extinction, it still remains a mystery bird. Currently, it is one of the rarest birds of the Old World and is critically endangered. Therefore, any information about recent records is of vital importance. Here, I report on the status of the species at one of its most important migrating stopover sites, the Danube delta, where promising observations were made in 2003 and 2004. As a consequence, plans are being made for follow-up studies and conservation activities. INTRODUCTION OBSERVATIONS OF SLENDER-BILLED CURLEWS DURING 2003–2004 The Danube delta, as well as the whole of the N and NW Black Sea coast is located on the main Slender-billed Cur- On 25 Jul 2003, I saw four Slender-billed Curlews together on lew migration route between its supposed W Siberian nest- the north-west end of the Taranova spit (45.443°N, 29.775°E) ing sites and its supposed Mediterranean wintering sites in the northern part of the delta between the channels Prorva (Gretton 1991; Heredia et al. -

Wildcat (Felis Silvestris Schreber, 1777) in Ukraine: Modern State of the Populations and Eastwards
WILDCAT (FELIS SILVESTRIS SCHREBER, 1777) IN UKRAINE: MODERN STATE OF THE POPULATIONS AND EASTWARDS... 233 UDC 599.742.73(477) WILDCAT (FELIS SILVESTRIS SCHREBER, 1777) IN UKRAINE: MODERN STATE OF THE POPULATIONS AND EASTWARDS EXPANSION OF THE SPECIES I. Zagorodniuk1, M. Gavrilyuk2, M. Drebet3, I. Skilsky4, A. Andrusenko5, A. Pirkhal6 1 National Museum of Natural History, NAS of Ukraine 15, Bohdan Khmelnytskyi St., Kyiv, 01601, Ukraine e-mail: [email protected] 2 Bohdan Khmelnitsky National University of Cherkasy 81, Shevchenko Blvd., Cherkasy 18031, Ukraine 3 National Nature Park “Podilski Tovtry” 6, Polskyi Rynok Sq., Kamianets-Podilskyi 32301, Khmelnytsk Region, Ukraine 4 Chernivtsi Regional Museum, 28, O. Kobylianska St., Chernivtsi 58002, Ukraine 5 National Nature Park “Bugsky Hard” 83, Pervomaiska St., Mygia 55223, Pervomaisky District, Mykolaiv Region, Ukraine 6 Vinnytsia Regional Laboratory Centre, 11, Malynovskyi St., Vinnytsya 21100, Ukraine Modern state of the wildcat populations in Ukraine is analyzed on the basis of de- tailed review and analysis of its records above (annotations) and before (detailed ca- dastre) 2000. Data on 71 modern records in 10 administrative regions of Ukraine are summarized, including: Lviv (8), Volyn (1), Ivano-Frankivsk (2), Chernivtsi (31), Khmelnyts kyi (4), Vinnytsia (14), Odesa (4), Mykolaiv (4), Kirovohrad (2) and Cherkasy (1) regions. Detailed maps of species distribution in some regions, and in Ukraine in gene ral, and the analysis of the rates of expansion as well as direction of change in species limits of the distribution are presented. Morphological characteristics of the samples from the territory of Ukraine are described. Keywords: wildcat, state of populations, geographic range, expansion, Ukraine. -

Management of Marine Protected Areas Management of Marine Protected Areas
Management of Marine Protected Areas Management of Marine Protected Areas A Network Perspective Edited by Paul D. Goriup This edition first published 2017 © 2017 John Wiley & Sons Ltd All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, except as permitted by law. Advice on how to obtain permission to reuse material from this title is available at www.wiley.com/go/permissions. The right of Paul D. Goriup to be identified as the author of the editorial material in this work has been asserted in accordance with law. Registered Office John Wiley & Sons Ltd, The Atrium, Southern Gate, Chichester, West Sussex, PO19 8SQ, UK Editorial Offices 111 River Street, Hoboken, NJ 07030, USA 9600 Garsington Road, Oxford, OX4 2DQ, UK The Atrium, Southern Gate, Chichester, West Sussex, PO19 8SQ, UK Boschstr. 12, 69469 Weinheim, Germany For details of our global editorial offices, customer services, and more information about Wiley products visit us at www.wiley.com. Wiley also publishes its books in a variety of electronic formats and by print‐on‐demand. Some content that appears in standard print versions of this book may not be available in other formats. Limit of Liability/Disclaimer of Warranty The publisher and the authors make no representations or warranties with respect to the accuracy or completeness of the contents of this work and specifically disclaim all warranties, including without limitation any implied warranties of fitness for a particular purpose. This work is sold with the understanding that the publisher is not engaged in rendering professional services. -

Project Mar Projet
IUCN Publications new scries N°5 Project Mar The conservation and management of temperate marshes, bogs and other wetlands SECOND VOLUME Projet Mar Conservation et aménagement des marécages, tourbières et autres milieux humides en zone tempérée DEUXIÈME VOLUME List of European and North African Wetlands of International Importance Liste des zones humides d'importance internationale en Europe et dans le Maghreb Project Mar The conservation and management of temperate marshes, bogs and other wetlands Projet Mar Conservation et aménagement des marécages, tourbières et autres milieux humides en zone tempérée Compiled by P. J. S. OLNEY and the staff of the IWRB/MAR Bureau Sponsored by IUCN, ICBP and IWRB Compilé par P. J. S. OLNEY et le personnel du Bureau BIRS/MAR Sous les auspices de l'UICN, du CIPO et du BIRS IUCN Publications new scries N°5 International Union for the Conservation of Nature and Natural Resources International Council for Bird Preservation International Wildfowl Research Bureau PROJECT MAR for the conservation and management of temperate wetlands List of European and North African wetlands of international importance Union Internationale pour la Conservation de la Nature et de ses Ressources Conseil International pour la Préservation des Oiseaux Bureau International de Recherches sur la Sauvagine PROJET MAR pour la conservation et l'aménagement des zones humides tempérées Liste des zones humides d'importance internationale en Europe et dans le Maghreb CONTENTS TABLE DES MATIERES Introduction (English) 7 Introduction (français) 13 Albania 21 Algérie 21 Austria 22 Belgique 24 Bulgaria 24 Czechoslovakia 25 Denmark 27 Espagne 30 Finland 33 France 34 Germany : Eastern part 41 Western part 43 Great Britain .