Bioresources.Com
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Effect of Enzymes on Strawberry Volatiles During Storage, at Different Ripeness
Effect of Enzymes on Strawberry Volatiles During Storage, at Different Ripeness Level, in Different Cultivars and During Eating Thesis Presented in Partial Fulfillment of the Requirements for the Degree Master of Science in the Graduate School of The Ohio State University By Gulsah Ozcan Graduate Program in Food Science and Technology The Ohio State University 2010 Thesis Committee: Sheryl Ann Barringer, Adviser W. James Harper John Litchfield 1 Copyright by Gülşah Özcan 2010 ii ABSTRACT Strawberry samples with enzyme activity and without enzyme activity (stannous chloride added) were measured for real time formation of lipoxygenase (LOX) derived aroma compounds after 5 min pureeing using selected ion flow tube mass spectrometry (SIFT-MS). The concentration of (Z)-3-hexenal and (E)-2-hexenal increased immediately after blending and gradually decreased over time while hexanal concentration increased for at least 5 min in ground strawberries. The formation of hexanal was slower than the formation of (Z)-3-hexenal and (E)-2-hexenal in the headspace of pureed strawberries. The concentration of LOX aldehydes and esters significantly increased during refrigerated storage. Damaging strawberries increased the concentration of LOX aldehydes but did not significantly affect the concentration of esters. The concentrations of many of the esters were strongly correlated to their corresponded acids and/or aldehydes. The concentration of LOX generated aldehydes decreased during ripening, while fruity esters increased. Different varieties had different aroma profiles and esters were the greatest percentage of the volatiles. The aroma release of some of the LOX derived aldehydes in the mouthspace in whole strawberries compared to chopped strawberries showed that these volatiles are formed in the mouth during chewing. -

Differences in Volatile Ester Composition Between Fragaria Ananassa and F
Scientia Horticulturae 150 (2013) 47–53 Contents lists available at SciVerse ScienceDirect Scientia Horticulturae journa l homepage: www.elsevier.com/locate/scihorti × Differences in volatile ester composition between Fragaria ananassa and F. ଝ vesca and implications for strawberry aroma patterns a,b b c b a,∗ Jing Dong , Yuntao Zhang , Xiaowei Tang , Wanmei Jin , Zhenhai Han a Institute for Horticultural Plants, College of Agronomy and Biotechnology, China Agricultural University, No. 2 Yuanmingyuan West Road, Haidian District, Beijing 100193, PR China b Institute of Forestry and Pomology, Beijing Academy of Agriculture and Forestry Sciences, No. 12 Ruiwangfen, Xiangshan, Haidian District, Beijing 100093, PR China c Beijing Vegetable Research Center, Beijing Academy of Agriculture and Forestry Sciences, Banjin, Haidian District, Beijing 100097, PR China a r t i c l e i n f o a b s t r a c t Article history: Esters are a very important component of strawberry (Fragaria sp.) aroma. In this study, fruit volatiles Received 8 April 2012 were isolated by solid-phase microextraction and analyzed by gas chromatography–mass spectrometry Received in revised form 1 November 2012 (SPME/GC–MS). It was found that F. × ananassa had more esters than F. vesca, but there was no significant Accepted 1 November 2012 difference in average relative content of esters. Twenty five esters were selected as predominant esters, 16 of which were prevalent in F. × ananassa and another 16 in F. vesca. Among them, hexyl acetate, Keywords: octyl acetate, ethyl butyrate, ethyl hexanoate, ethyl octanoate, methyl decanoate, and ethyl decanoate Volatile ester composition were found in both F. -

Expanding the Modular Ester Fermentative Pathways for Combinatorial Biosynthesis of Esters from Volatile Organic Acids
ARTICLE Expanding the Modular Ester Fermentative Pathways for Combinatorial Biosynthesis of Esters From Volatile Organic Acids Donovan S. Layton,1,2 Cong T. Trinh1,2,3 1 Department of Chemical and Biomolecular Engineering, University of Tennessee, Knoxville, Tennessee 2 BioEnergy Science Center (BESC), Oak Ridge National Laboratory, Oak Ridge, Tennessee 3 Bredesen Center for Interdisciplinary Research and Graduate Education, University of Tennessee, Knoxville, Tennessee; telephone: þ865-974-8121; fax: 865-974-7076; e-mail: [email protected] Biotechnol. Bioeng. 2016;113: 1764–1776. ABSTRACT: Volatile organic acids are byproducts of fermentative ß 2016 Wiley Periodicals, Inc. metabolism, for example, anaerobic digestion of lignocellulosic KEYWORDS: modular chassis cell; carboxylate; ester; acyl acetate; biomass or organic wastes, and are often times undesired inhibiting acyl acylate; ester fermentative pathway cell growth and reducing directed formation of the desired products. Here, we devised a general framework for upgrading these volatile organic acids to high-value esters that can be used as flavors, fragrances, solvents, and biofuels. This framework employs the acid-to-ester modules, consisting of an AAT (alcohol Introduction acyltransferase) plus ACT (acyl CoA transferase) submodule and an alcohol submodule, for co-fermentation of sugars and organic Harnessing renewable or waste feedstocks (e.g., switchgrass, corn acids to acyl CoAs and alcohols to form a combinatorial library of stover, agricultural residue, or municipal solid waste) -

University Microfilms, Inc., Ann Arbor, Michigan BIOCHEMICAL STUDIES of ZOOGLOEA RAMIGERA ISOLATE 115
This dissertation has been microfilmed exactly as received 69-11,658 JOYCE, Gayle Hahn, 1937- BIOCHEMICAL STUDIES OF ZOOGLOEA RAMIGERA ISOLATE 115 WITH EMPHASIS ON ETHYL ALCOHOL METABOLISM. The Ohio State University, Ph.D., 1968 Microbiology University Microfilms, Inc., Ann Arbor, Michigan BIOCHEMICAL STUDIES OF ZOOGLOEA RAMIGERA ISOLATE 115 WITH EMPHASIS ON ETHYL ALCOHOL METABOLISM DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University v * By Gayle hT Joyce, B.S., M.S. ****** The Ohio State University 1968 Approved by / Adviser ' fj . qpobiAcademic Faculty of Miqpobial.qpobiAcademic and Cellular Biology ACKNOWLEDGMENTS The author wishes to express her sincere gratitude to Dr. Patrick R. Dugan for the many helpful suggestions and guidance given during the course of this study and in the preparation of this manuscript. Her appreciation is expressed to the graduate students of the Faculty of Microbial and Cellular Biology, particularly to Mr. Jon Tuttle, for their helpful discussions and assistance, to Mrs. John Olenzak for her technical assistance, and to Mr. Robert Joyce for his patience during the course of this study. The use of the facilities of the Water Resources Center of The Ohio State University is appreciated. The research was supported in part by Research Grant No. WP-00713 from the Federal Water Pollution Control Administration, U.S. Department of the Interior. VITA September 10, 1937 Born - Cleveland, Ohio 1959 ....... B.S., Kent State University, Kent, Ohio 1959-1961 ........ Research Assistant, Department of Microbiology, The Pennsylvania State University, University Park, Pennsylvania 1961 ...... -

Product Specification
Product Specification Product Name: ETHYL BUTYRATE NATURAL Vigon Code: 500132 CAS Number: 105-54-4 FEMA Number: 2427 Characteristic: Specification: Acid Value 0.000 1.000 Purity % 98.00 100.00 Gas Chromatograph PASSES TEST Odor POWERFUL, FRUITY, BANANA-LIKE ODOR. Refractive Index (@20c) 1.3910 1.3940 Specific Gravity (@25c) 0.8700 0.8770 Color/appearance COLORLESS LIQUID. Additional Product Information: Flash Point: 75 °F Shelf Life: 36 Months Storage Conditions: Stable when stored in its original package, tightly sealed in a cool (46-90ºF) and dry location out of direct heat and light. Effective Date: September 11, 2012 Printed Date:September 8, 2021 Vigon International, LLC. 127 Airport Road East Stroudsburg, PA 18301-9629 USA | Tel: +1 570-476-6300 | Fax: +1 570-476-1110 | Email: [email protected] | Web: vigon.com SAFETY DATA SHEET 500132 ETHYL BUTYRATE NATURAL Revision Date: 05-17-2021 Page 1 of 11 Version # 06 Print Date: 05-17-2021 1. IDENTIFICATION Product Description: ETHYL BUTYRATE NATURAL CAS # 105-54-4 FEMA Number 2427 Other means of identification Vigon Item # 500132 Recommended use Concentrated aromatic and flavor ingredient which may be used in flavor and fragrance compounds according to legal and IFRA or FEMA GRAS/FDA guidelines. Recommended restrictions For Manufacturing Use Only Company 24 Hour Emergency Response Information Vigon International, Inc. INFOTRAC (ACCT# 78928); 127 Airport Road 1-800-535-5053 WITHIN THE U.S.A. 1-352-323-3500 OUTSIDE THE U.S.A. E. Stroudsburg, PA 18301 For information call: 570-476-6300 Web Site: www.vigon.com Manufacturer/Importer/Supplier/Distributor information Manufacturer Company name Vigon International, Inc. -

Response Surface Methodology Approach for the Synthesis of Ethyl Butyrate
V.C. ARAGÃO et al.: Synthesis of Ethyl Butyrate, Food Technol. Biotechnol. 49 (1) 103–110 (2011) 103 ISSN 1330-9862 original scientific paper (FTB-2253) Response Surface Methodology Approach for the Synthesis of Ethyl Butyrate Vitor Cardoso Aragão, Maria Rita Alaniz Porto, Carlos André Veiga Burkert, Susana Juliano Kalil and Janaína Fernandes de Medeiros Burkert* Department of Chemistry, Federal University Foundation of Rio Grande, P.O. Box 474, 96201-900, Rio Grande, RS, Brazil Received: March 13, 2009 Accepted: May 27, 2010 Summary Response surface methodology was used to determine optimum conditions for the esterification of ethanol and butyric acid to produce a flavour ester using immobilized li- pase. Various reaction parameters including butyric acid concentration, enzyme concentra- tion, temperature and ethanol/butyric acid molar ratio affecting ethyl butyrate production were investigated using a fractional factorial design 24–1. Based on the results from the first factorial design, all of the variables which were significant in the process were selected to be used in a 24 central composite rotatable design (CCRD). The optimum conditions for the enzymatic reaction were obtained at a 90 mM butyric acid concentration using a 7.7 g/L enzyme concentration at 45 °C and the ethanol/butyric acid molar ratio of 1:1 for 3 h. The esterification percentage, under these conditions, was 87 %. Key words: ethyl butyrate, immobilized lipase, esterification, optimization, enzymatic syn- thesis Most of the commercial esters can be obtained di- Introduction rectly by extraction from plant materials, but the high cost and low quantity of the obtained product make this Flavour is usually the result of the presence, within technique inadequate for industrial applications. -

Cigarette Additives, Carcinogens and Chemicals Nicotine
Cigarette Additives, Carcinogens and Chemicals Nicotine A Destructive Natural Pesticide Which ... Is extremely addictive when smoked Is extremely addictive when chewed Causes addiction as permanent as Is harder to quit than heroin or cocaine alcoholism Is not medicine and its use not therapy Is ineffective as a stand-alone quitting aid Prevents pre-cancerous cells from dying Accelerates cancer tumor growth rates Contributes to artery hardening Has a metabolite which may cause cancer May kill brain cells and impair memory Is linked to lung cancer Likely causes brain damage and Is also a fetus destroying teratogen depression Kills half of adult smokers 13-14 years Is beat by never taking another puff or early chew! 81 Cancer Causing Chemicals Have So Far Been Identified in Cigarettes Acetaldehyde Acetamide Acrylamide Acrylonitrile 2-Amino-3,4-dimethyl-3H-imidazo[4,5-f]quinoline (MeIQ) 3-Amino-1,4-dimethyl-5H-pyrido [4,3-b]indole (Trp-P-1) 2-Amino-l-methyl-6-phenyl-1H-imidazo [4,5-b]pyridine (PhlP) 2-Amino-6-methyldipyrido[1,2-a:3',2'-d]imidazole (Glu-P-1) 3-Amino-l-methyl-5H-pyrido {4,3-b]indole (Trp-P-2 2-Amino-3-methyl-9H-pyrido[2,3-b]indole (MeAaC) 2-Amino-9H-pyrido[2,3-b]indole (AaC) 4-Aminobiphenyl 2-Aminodipyrido[1,2-a:3',2'-d]imidazole (Glu-P-2) 0-Anisidine Arsenic Benz[a]anthracene Benzene Benzo[a]pyrene Benzo[b]fluoranthene Benzo[j]fluoranthene Benzo[k]fluoranthene Benzo[b]furan Beryllium 1,3-Butadiene Cadmium Catechol (1,2-benzenediol) p-Chloroaniline Chloroform Cobalt p,p'-DDT Dibenz[a,h]acridine Dibenz[a,j]acridine Dibenz(a,h)anthracene -

Evaluation of Perceptual Interactions Between Ester Aroma Components in Langjiu by GC-MS, GC-O, Sensory Analysis, and Vector Model
foods Article Evaluation of Perceptual Interactions between Ester Aroma Components in Langjiu by GC-MS, GC-O, Sensory Analysis, and Vector Model Yunwei Niu 1, Ying Liu 1 and Zuobing Xiao 1,2,* 1 School of Perfume and Aroma Technology, Shanghai Institute of Technology, No.100 Haiquan Road, Shanghai 201418, China; [email protected] (Y.N.); [email protected] (Y.L.) 2 Beijing Advanced Innovation Center for Food Nutrition and Human Health, No. 11/33, Fucheng Road, Haidian District, Beijing 100048, China * Correspondence: [email protected]; Tel.: +86-21-60873511 Received: 3 January 2020; Accepted: 11 February 2020; Published: 13 February 2020 Abstract: The volatile compounds of three Langjiu (“Honghualangshi, HHL”, “Zhenpinlang, ZPL”, and “Langpailangjiu, LPLJ”) were studied by gas chromatography-olfactometry (GC-O) and gas chromatography-mass spectrometry (GC-MS). The results showed that a total of 31, 30, and 30 ester compounds making a contribution to aroma were present in the HHL, ZPL, and LPLJ samples, respectively. From these esters, 16 compounds were identified as important odour substances, and their odour activity values (OAVs) were greater than 1. The key ester components were selected as: ethyl acetate, ethyl 2-methylbutyrate, ethyl 3-methyl butyrate, ethyl hexanoate, and ethyl phenylacetate by aroma extract dilution analysis (AEDA), odour activity value (OAV), and omission testing. Five esters were studied for perceptual interactions while using Feller’s additive model, OAV, and a vector model. Among these mixtures, they all have an enhancing or synergistic effect. Among these mixtures, one mixture presented an additive effect and nine mixtures showed a synergistic effect. -
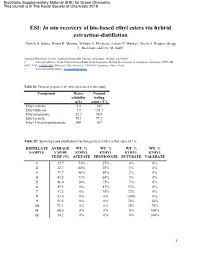
In Situ Recovery of Bio-Based Ethyl Esters Via Hybrid Extraction-Distillation
Electronic Supplementary Material (ESI) for Green Chemistry. This journal is © The Royal Society of Chemistry 2019 ESI: In situ recovery of bio-based ethyl esters via hybrid extraction-distillation Patrick O. Saboe, Hanna R. Monroe, William E. Michener, Lorenz P. Manker†, Stefan J. Haugen, Gregg T. Beckham, and Eric M. Karp‡ National Bioenergy Center, National Renewable Energy Laboratory, Golden, CO 80401 † Current address: Ecole Polytechnique Fédérale de Lausanne, Institut des sciences et ingénierie chimiques, EPFL SB ISIC LPDC, CH H2 545 (Bâtiment CH), Station 6, CH-1015, Lausanne, Switzerland ‡ Corresponding author: [email protected] Table S1. Physical properties of ethyl esters used in this study Compound Water Normal solubility boiling (g/L) point (°C) Ethyl valerate 2.2 145 Ethyl butyrate 7.9 121.1 Ethyl propionate 22.2 98.9 Ethyl acetate 78.1 77.1 Ethyl 3-hydroxypropanoate 409 187 Table S2. Spinning band distillation fraction purities with a reflux ratio of 1.0 DISTILLATE AVERAGE WT. % WT. % WT. % WT. % SAMPLE VAPOR ETHYL ETHYL ETHYL ETHYL TEMP (°C) ACETATE PROPIONATE BUTYRATE VALERATE 1 25.7 73% 27% 0% 0% 2 32.7 64% 35% 1% 0% 3 37.7 48% 50% 2% 0% 4 43.2 31% 64% 5% 0% 5 46.0 18% 75% 7% 0% 6 49.5 0% 87% 13% 0% 7 53.2 0% 78% 22% 0% 8 52.6 0% 0% 100% 0% 9 51.5 0% 0% 74% 26% 10 71.1 0% 0% 25% 75% 11 68.6 0% 0% 0% 100% 12 54.2 0% 0% 0% 100% 1 Fig. -

Studies of Ester Formation on a Cellulose Matrix
2006:18 LICENTIATE T H E SI S Studies of Ester Formation on a Cellulose Matrix Anna Pantze Luleå University of Technology LTU Skellefteå Division of Wood Science and Technology 2006:18|: 102-1757|: -c -- 06 ⁄18 -- LICENTIATE THESIS Studies of Ester Formation on a Cellulose Matrix Anna Pantze Luleå University of Technology Div. of Wood Science and Technology LTU Skellefteå 931 87 Skellefteå, Sweden i ii ABSTRACT In this work a model system has been developed to quantitatively study esterification of cellulosic material during drying and heating in which solid-state conditions can be said to predominate. The formation of ester cross-links in cellulosic or lignocellulosic composite materials during drying and heating is of interest for production of paper and wood composite boards, for wood modification and in giving cotton materials improved characteristics. The results support the hypothesis that direct ester formation, without anhydride intermediate, occurs between carboxylic acids of suitable structure and cellulosic hydroxyls at elevated temperatures even after such short reaction times as 5 minutes. Ester formation has been observed at temperatures ranging from 90°C to 180°C. Another important conclusion is that a hydroxy or keto group in alpha position of the carboxylic acids seems to favour ester formation. A critical parameter for ester formation is pH, and the results indicate that pH < 2 strongly favours esterification reactions, whereas almost no esters are produced at pH > 5–6. In the experiments, low moisture content, higher temperatures and longer reaction times also favour ester formation, with some exceptions. Another interesting finding is that three pyrrolidine structures are produced after esterification and aminolysis of 2-ketobutyric acid. -

Mechanisms of the Regulation of Ester Balance Between Oxidation And
www.nature.com/scientificreports OPEN Mechanisms of the regulation of ester balance between oxidation and esterifcation in aged Baijiu Yonghui Deng1, Ayuan Xiong1, Kun Zhao1, Yaru Hu1, Bisheng Kuang2, Xiang Xiong2, Zhilong Yang2, Yougui Yu1,2,3* & Qing Zheng1,2,3* The infuence of oxidation and esterifcation on the ester balance of aged Baijiu and methodology for the rational design of liquor favors to optimize the practice of Baijiu production are not completely understood. We investigated the infuence of ester balance on the favor compounds of aged Baijiu by conducting constituent analysis of Baijiu from diferent aging times (0, 1, 2, 3, 4, 5, and 10 years). The changes of the main favor compounds in the aging process were determined, and the correlations among diferent chemical reactions, such as oxidation, hydrolysis, and esterifcation, were systematically expounded. Furthermore, cluster analysis of the heat map indicated signifcant diferences between aged Baijiu and new Baijiu and recommended a suitable aging time of 2–3 years. Baijiu is a typical Chinese liquor originating from the Western Han Dynasty, which plays an important role in practical life in China and constitutes a unique position in the liquor industry with an annual production value of approximately 52 billion dollars 1,2. In recent decades, with the wide application of chromatographic analysis technology, the chemistry behind Baijiu brewing has developed rapidly, providing the scientifc basis for Baijiu production3,4. However, the production process in practice is not fully established because many factors infuence Baijiu production5. Te diferences in raw materials, fermentation, distillation, aging, and other processes will have a direct efect on product quality, leading to product governance instability, which hinders the development of the Baijiu industry6–8. -

Distilled Alcoholic Beverage Production Using Reactive Distillation Techniques
DISTILLED ALCOHOLIC BEVERAGE PRODUCTION USING REACTIVE DISTILLATION TECHNIQUES By Jacob David Rochte A DISSERTATION Submitted to Michigan State University in partial fulfillment of the requirements for the degree of Chemical Engineering – Doctor of Philosophy 2018 ABSTRACT DISTILLED ALCOHOLIC BEVERAGE PRODUCTION USING REACTIVE DISTILLATION TECHNIQUES By Jacob David Rochte The goal of this research was to couple industrial beverage distillation with a chemical reaction to create a flavored spirit without adding anything after the final distillation. Ethanol reacted with butyric acid in the final distillation over Amberlyst® 15 to create ethyl butyrate, which gave the spirit a fruity smell comparable to Juicy Fruit® gum. It was shown that if a beverage ethanol fermentation having 63±3 g/L ethanol and 1 g/L butyric acid were distilled, the low wine will have 357 ± 32 g/L ethanol and 1:4 ± 0:1 g/L butyric acid. This sets up the low wine to be distilled over the catalyst to create the ethyl butyrate. For the study, low wine was obtained from a craft distillery. This gave the most accurate representation of how this system would act in the beverage industry. Butyric acid was added to this low wine and distilled on a glass vigreux column with copper wire on the inside to simulate an industrial copper distillation column. The distillations started with a pot ethanol concentration of 30% ABV and varied: the butyric acid starting concentration from 0.5 g/L to 5 g/L, the catalyst loading from 1 g dry / L to 100 g dry/L, and the catalyst position in the column from being in the pot, the bottom of the column, and the top of the column.