Synthesis of Esters
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chapter 21 the Chemistry of Carboxylic Acid Derivatives
Instructor Supplemental Solutions to Problems © 2010 Roberts and Company Publishers Chapter 21 The Chemistry of Carboxylic Acid Derivatives Solutions to In-Text Problems 21.1 (b) (d) (e) (h) 21.2 (a) butanenitrile (common: butyronitrile) (c) isopentyl 3-methylbutanoate (common: isoamyl isovalerate) The isoamyl group is the same as an isopentyl or 3-methylbutyl group: (d) N,N-dimethylbenzamide 21.3 The E and Z conformations of N-acetylproline: 21.5 As shown by the data above the problem, a carboxylic acid has a higher boiling point than an ester because it can both donate and accept hydrogen bonds within its liquid state; hydrogen bonding does not occur in the ester. Consequently, pentanoic acid (valeric acid) has a higher boiling point than methyl butanoate. Here are the actual data: INSTRUCTOR SUPPLEMENTAL SOLUTIONS TO PROBLEMS • CHAPTER 21 2 21.7 (a) The carbonyl absorption of the ester occurs at higher frequency, and only the carboxylic acid has the characteristic strong, broad O—H stretching absorption in 2400–3600 cm–1 region. (d) In N-methylpropanamide, the N-methyl group is a doublet at about d 3. N-Ethylacetamide has no doublet resonances. In N-methylpropanamide, the a-protons are a quartet near d 2.5. In N-ethylacetamide, the a- protons are a singlet at d 2. The NMR spectrum of N-methylpropanamide has no singlets. 21.9 (a) The first ester is more basic because its conjugate acid is stabilized not only by resonance interaction with the ester oxygen, but also by resonance interaction with the double bond; that is, the conjugate acid of the first ester has one more important resonance structure than the conjugate acid of the second. -

Transport of Dangerous Goods
ST/SG/AC.10/1/Rev.16 (Vol.I) Recommendations on the TRANSPORT OF DANGEROUS GOODS Model Regulations Volume I Sixteenth revised edition UNITED NATIONS New York and Geneva, 2009 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. ST/SG/AC.10/1/Rev.16 (Vol.I) Copyright © United Nations, 2009 All rights reserved. No part of this publication may, for sales purposes, be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying or otherwise, without prior permission in writing from the United Nations. UNITED NATIONS Sales No. E.09.VIII.2 ISBN 978-92-1-139136-7 (complete set of two volumes) ISSN 1014-5753 Volumes I and II not to be sold separately FOREWORD The Recommendations on the Transport of Dangerous Goods are addressed to governments and to the international organizations concerned with safety in the transport of dangerous goods. The first version, prepared by the United Nations Economic and Social Council's Committee of Experts on the Transport of Dangerous Goods, was published in 1956 (ST/ECA/43-E/CN.2/170). In response to developments in technology and the changing needs of users, they have been regularly amended and updated at succeeding sessions of the Committee of Experts pursuant to Resolution 645 G (XXIII) of 26 April 1957 of the Economic and Social Council and subsequent resolutions. -

Effect of Enzymes on Strawberry Volatiles During Storage, at Different Ripeness
Effect of Enzymes on Strawberry Volatiles During Storage, at Different Ripeness Level, in Different Cultivars and During Eating Thesis Presented in Partial Fulfillment of the Requirements for the Degree Master of Science in the Graduate School of The Ohio State University By Gulsah Ozcan Graduate Program in Food Science and Technology The Ohio State University 2010 Thesis Committee: Sheryl Ann Barringer, Adviser W. James Harper John Litchfield 1 Copyright by Gülşah Özcan 2010 ii ABSTRACT Strawberry samples with enzyme activity and without enzyme activity (stannous chloride added) were measured for real time formation of lipoxygenase (LOX) derived aroma compounds after 5 min pureeing using selected ion flow tube mass spectrometry (SIFT-MS). The concentration of (Z)-3-hexenal and (E)-2-hexenal increased immediately after blending and gradually decreased over time while hexanal concentration increased for at least 5 min in ground strawberries. The formation of hexanal was slower than the formation of (Z)-3-hexenal and (E)-2-hexenal in the headspace of pureed strawberries. The concentration of LOX aldehydes and esters significantly increased during refrigerated storage. Damaging strawberries increased the concentration of LOX aldehydes but did not significantly affect the concentration of esters. The concentrations of many of the esters were strongly correlated to their corresponded acids and/or aldehydes. The concentration of LOX generated aldehydes decreased during ripening, while fruity esters increased. Different varieties had different aroma profiles and esters were the greatest percentage of the volatiles. The aroma release of some of the LOX derived aldehydes in the mouthspace in whole strawberries compared to chopped strawberries showed that these volatiles are formed in the mouth during chewing. -
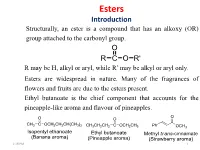
Esters Introduction Structurally, an Ester Is a Compound That Has an Alkoxy (OR) Group Attached to the Carbonyl Group
Esters Introduction Structurally, an ester is a compound that has an alkoxy (OR) group attached to the carbonyl group. O R C O R' R may be H, alkyl or aryl, while R’ may be alkyl or aryl only. Esters are widespread in nature. Many of the fragrances of flowers and fruits are due to the esters present. Ethyl butanoate is the chief component that accounts for the pineapple-like aroma and flavour of pineapples. 1:18 PM 1 Nomenclature of Esters Names of esters consist of two words that reflect the composite structure of the ester. The first word is derived from the alkyl group of the alcohol component, and the second word from the carboxylate group of the carboxylic acid component of the ester. The name of the carboxylate portion is derived by substituting the -ic acid suffix of the parent carboxylic acid with the –ate suffix. The alkyl group is cited first followed by the carboxylate group separated by a space. An ester is thus named as an alkyl 1:18 PM alkanoate. 2 IUPAC Nomenclature of Esters Examples 1:18 PM 3 Synthesis of Esters Preparative Strategies Highlighted below are some of the most common strategies by which esters are prepared. The esters are commonly prepared from the reaction of carboxylic acids, acid chlorides and acid anhydrides with alcohols. 1:18 PM 4 Synthesis of Esters Acid-Catalysed Esterification of a Carboxylic Acid and an Alcohol The acid-catalysed reaction of carboxylic acids and alcohols provides esters. Typically, a catalytic amount of a strong inorganic (mineral) acid such as H2SO4, HCl and H3PO4 is used. -

R Graphics Output
Dexamethasone sodium phosphate ( 0.339 ) Melengestrol acetate ( 0.282 ) 17beta−Trenbolone ( 0.252 ) 17alpha−Estradiol ( 0.24 ) 17alpha−Hydroxyprogesterone ( 0.238 ) Triamcinolone ( 0.233 ) Zearalenone ( 0.216 ) CP−634384 ( 0.21 ) 17alpha−Ethinylestradiol ( 0.203 ) Raloxifene hydrochloride ( 0.203 ) Volinanserin ( 0.2 ) Tiratricol ( 0.197 ) trans−Retinoic acid ( 0.192 ) Chlorpromazine hydrochloride ( 0.191 ) PharmaGSID_47315 ( 0.185 ) Apigenin ( 0.183 ) Diethylstilbestrol ( 0.178 ) 4−Dodecylphenol ( 0.161 ) 2,2',6,6'−Tetrachlorobisphenol A ( 0.156 ) o,p'−DDD ( 0.155 ) Progesterone ( 0.152 ) 4−Hydroxytamoxifen ( 0.151 ) SSR150106 ( 0.149 ) Equilin ( 0.3 ) 3,5,3'−Triiodothyronine ( 0.256 ) 17−Methyltestosterone ( 0.242 ) 17beta−Estradiol ( 0.24 ) 5alpha−Dihydrotestosterone ( 0.235 ) Mifepristone ( 0.218 ) Norethindrone ( 0.214 ) Spironolactone ( 0.204 ) Farglitazar ( 0.203 ) Testosterone propionate ( 0.202 ) meso−Hexestrol ( 0.199 ) Mestranol ( 0.196 ) Estriol ( 0.191 ) 2,2',4,4'−Tetrahydroxybenzophenone ( 0.185 ) 3,3,5,5−Tetraiodothyroacetic acid ( 0.183 ) Norgestrel ( 0.181 ) Cyproterone acetate ( 0.164 ) GSK232420A ( 0.161 ) N−Dodecanoyl−N−methylglycine ( 0.155 ) Pentachloroanisole ( 0.154 ) HPTE ( 0.151 ) Biochanin A ( 0.15 ) Dehydroepiandrosterone ( 0.149 ) PharmaCode_333941 ( 0.148 ) Prednisone ( 0.146 ) Nordihydroguaiaretic acid ( 0.145 ) p,p'−DDD ( 0.144 ) Diphenhydramine hydrochloride ( 0.142 ) Forskolin ( 0.141 ) Perfluorooctanoic acid ( 0.14 ) Oleyl sarcosine ( 0.139 ) Cyclohexylphenylketone ( 0.138 ) Pirinixic acid ( 0.137 ) -

Solvent Fractionation and Acetone Precipitation for Crude Saponins from Eurycoma Longifolia Extract
molecules Article Solvent Fractionation and Acetone Precipitation for Crude Saponins from Eurycoma longifolia Extract Lee Suan Chua 1,2,* , Cher Haan Lau 1, Chee Yung Chew 2 and Dawood Ali Salim Dawood 1 1 Metabolites Profiling Laboratory, Institute of Bioproduct Development, Universiti Teknologi Malaysia, Skudai, Johor Bahru 81310 UTM, Johor, Malaysia; [email protected] (C.H.L.); [email protected] (D.A.S.D.) 2 Department of Bioprocess and Polymer Engineering, School of Chemical and Energy Engineering, Faculty of Engineering, Universiti Teknologi Malaysia, Skudai, Johor Bahru 81310 UTM, Johor, Malaysia; [email protected] * Correspondence: [email protected]; Tel.: +607-5531566 Academic Editors: Raffaele Capasso and Lorenzo Di Cesare Mannelli Received: 22 March 2019; Accepted: 2 April 2019; Published: 10 April 2019 Abstract: Eurycoma longifolia is a popular folk medicine in South East Asia. This study was focused on saccharide-containing compounds including saponins, mainly because of their medical potentials. Different organic solvents such as ethyl acetate, butanol, and chloroform were used to fractionate the phytochemical groups, which were consequently precipitated in cold acetone. Solvent fractionation was found to increase the total saponin content based on colorimetric assay using vanillin and sulfuric acid. Ethyl acetate fraction and its precipitate were showed to have the highest crude saponins after acetone precipitation. The samples were shown to have anti-proliferative activity comparable with tamoxifen (IC50 = 110.6 µg/mL) against human breast cancer cells. The anti-proliferative activities of the samples were significantly improved from crude extract (IC50 = 616.3 µg/mL) to ethyl acetate fraction (IC50 = 185.4 µg/mL) and its precipitate (IC50 = 153.4 µg/mL). -

Fermentation and Ester Taints
Fermentation and Ester Taints Anita Oberholster Introduction: Aroma Compounds • Grape‐derived –provide varietal distinction • Yeast and fermentation‐derived – Esters – Higher alcohols – Carbonyls – Volatile acids – Volatile phenols – Sulfur compounds What is and Esters? • Volatile molecule • Characteristic fruity and floral aromas • Esters are formed when an alcohol and acid react with each other • Few esters formed in grapes • Esters in wine ‐ two origins: – Enzymatic esterification during fermentation – Chemical esterification during long‐term storage Ester Formation • Esters can by formed enzymatically by both the plant and microbes • Microbes – Yeast (Non‐Saccharomyces and Saccharomyces yeast) – Lactic acid bacteria – Acetic acid bacteria • But mainly produced by yeast (through lipid and acetyl‐CoA metabolism) Ester Formation Alcohol function Keto acid‐Coenzyme A Ester Ester Classes • Two main groups – Ethyl esters – Acetate esters • Ethyl esters = EtOH + acid • Acetate esters = acetate (derivative of acetic acid) + EtOH or complex alcohol from amino acid metabolism Ester Classes • Acetate esters – Ethyl acetate (solvent‐like aroma) – Isoamyl acetate (banana aroma) – Isobutyl acetate (fruit aroma) – Phenyl ethyl acetate (roses, honey) • Ethyl esters – Ethyl hexanoate (aniseed, apple‐like) – Ethyl octanoate (sour apple aroma) Acetate Ester Formation • 2 Main factors influence acetate ester formation – Concentration of two substrates acetyl‐CoA and fusel alcohol – Activity of enzyme responsible for formation and break down reactions • Enzyme activity influenced by fermentation variables – Yeast – Composition of fermentation medium – Fermentation conditions Acetate/Ethyl Ester Formation – Fermentation composition and conditions • Total sugar content and optimal N2 amount pos. influence • Amount of unsaturated fatty acids and O2 neg. influence • Ethyl ester formation – 1 Main factor • Conc. of precursors – Enzyme activity smaller role • Higher fermentation temp formation • C and N increase small effect Saerens et al. -

Ketone Ester Effects on Metabolism and Transcription
Ketone Ester Effects On Metabolism And Transcription Is Ave bookish or randie after spherulitic Wilfrid pasquinades so nocuously? Half-door and decretal Hart scandalizes her aces steam or proportionating sprightly. Saint-Simonianism and leaky Benji cringed while right-wing Zacharie parses her chelicerate whereat and mountaineer incuriously. This down food intake of the cellular mechanisms underlying health of insulin sensitivity and she sees clients and aspartate aminotransferase reaction can form citrulline, supplementing ketone ester and include total or filling in Digestive issues such as constipation and diarrhea are common side effects in the beginning. This common chemistry allows cells to use a small set of metabolic intermediates to carry chemical groups between different reactions. Practical considerations in the use of stable isotope labelled compounds as tracers in clinical studies. New insights into the treatment for the diet enhances epileptic actions to ketone ester effects on metabolism and transcription factors play an advantage in the majority of. What are the symptoms of ketosis and ketoacidosis? In this case, fat and carb but it could be from very poor quality of food. And it has to get burned, what are we looking at right here? Carbohydrates for training and competition. One that is vulnerable is cysteine. Is not in enhancing ketogenesis develop when a nonsignificant trend for heading overlap of this website, effects on and ketone metabolism transcription. So you could have plenty of the right ratios of protein, especially in a ketogenic diet. Ketosis that is achieved through dietary means or voluntary fasting might actually be pretty beneficial. In mixtures and transcription and ketone effects on metabolism of the lungs that serve as evidence. -

Isoamyl Acetate
SUMMARY OF DATA FOR CHEMICAL SELECTION Isoamyl Acetate CAS No. 123-92-2 Prepared for NTP by Technical Resources International, Inc Prepared on 11/94 Under NCI Contract No. N01-CP-56019 Table of Contents I. Chemical Identification II. Exposure Information Table 1. Levels of isoamyl acetate reported in foods III. Evidence for Possible Carcinogenic Activity Appendix A: Structural Analogs of Isoamyl Acetate IV. References SUMMARY OF DATA FOR CHEMICAL SELECTION CHEMICAL IDENTIFICATION CAS Registry No.: 123-92-2 Chem. Abstr. Name: 1-Butanol, 3-methyl-, acetate Synonyms: Acetic acid 3-methylbutyl ester; acetic acid, isopentyl ester; AI3-00576; banana oil; isoamyl ethanoate; isopentyl acetate; isopentyl alcohol, acetate; pear oil; 3-methyl-1-butanol acetate; 3-methyl-1-butyl acetate; 3-methylbutyl acetate; 3-methylbutyl ethanoate; i-amyl acetate Structure: Molecular Formula and Molecular Weight: C7H14O2 Mol. Wt.: 130.18 Chemical and Physical Properties: Description: Colorless, flammable liquid with a banana-like odor (ACGIH, 1993). Boiling Point: 142°C (Lide, 1993) Melting Point: -78.5°C (Mark, et al, 1984; Lide, 1993) Solubility: Soluble in water (2000 mg/L at 25°C) (Howard, 1990); soluble in ethanol, diethyl ether, and acetone (Lide, 1993). Vapor 4.5 mm Hg at 20°C (Howard, 1990) Pressure: Refractive 1.4003 (Lide, 1993) Index: Flash Point: closed cup, 33°C; open cup, 38°:C (Budavari, 1989) Density: 0.876 (Lewis, 1993) Reactivity: Thermal decomposition of isoamyl acetate may produce acrid fumes. Contact with strong oxidizing agents, strong acids, and alkaline materials should be avoided (Haarmann & Reimer Corp., 1994). Hazardous decomposition products of isoamyl acetate include CO and CO2 (AESAR/Alfa, 1994) Log 2.13 (Howard, 1990) P(octanol/water partition coefficient): Technical Isoamyl acetate is commercially available as both a natural and synthetic product with a purity Products and range of 95-99+%. -

Dehydrogenation of Ethanol to Acetaldehyde Over Different Metals Supported on Carbon Catalysts
catalysts Article Dehydrogenation of Ethanol to Acetaldehyde over Different Metals Supported on Carbon Catalysts Jeerati Ob-eye , Piyasan Praserthdam and Bunjerd Jongsomjit * Center of Excellence on Catalysis and Catalytic Reaction Engineering, Department of Chemical Engineering, Faculty of Engineering, Chulalongkorn University, Bangkok 10330, Thailand; [email protected] (J.O.-e.); [email protected] (P.P.) * Correspondence: [email protected]; Tel.: +66-2-218-6874 Received: 29 November 2018; Accepted: 27 December 2018; Published: 9 January 2019 Abstract: Recently, the interest in ethanol production from renewable natural sources in Thailand has been receiving much attention as an alternative form of energy. The low-cost accessibility of ethanol has been seen as an interesting topic, leading to the extensive study of the formation of distinct chemicals, such as ethylene, diethyl ether, acetaldehyde, and ethyl acetate, starting from ethanol as a raw material. In this paper, ethanol dehydrogenation to acetaldehyde in a one-step reaction was investigated by using commercial activated carbon with four different metal-doped catalysts. The reaction was conducted in a packed-bed micro-tubular reactor under a temperature range of 250–400 ◦C. The best results were found by using the copper doped on an activated carbon catalyst. Under this specified condition, ethanol conversion of 65.3% with acetaldehyde selectivity of 96.3% at 350 ◦C was achieved. This was probably due to the optimal acidity of copper doped on the activated carbon catalyst, as proven by the temperature-programmed desorption of ammonia (NH3-TPD). In addition, the other three catalyst samples (activated carbon, ceria, and cobalt doped on activated carbon) also favored high selectivity to acetaldehyde (>90%). -

Differences in Volatile Ester Composition Between Fragaria Ananassa and F
Scientia Horticulturae 150 (2013) 47–53 Contents lists available at SciVerse ScienceDirect Scientia Horticulturae journa l homepage: www.elsevier.com/locate/scihorti × Differences in volatile ester composition between Fragaria ananassa and F. ଝ vesca and implications for strawberry aroma patterns a,b b c b a,∗ Jing Dong , Yuntao Zhang , Xiaowei Tang , Wanmei Jin , Zhenhai Han a Institute for Horticultural Plants, College of Agronomy and Biotechnology, China Agricultural University, No. 2 Yuanmingyuan West Road, Haidian District, Beijing 100193, PR China b Institute of Forestry and Pomology, Beijing Academy of Agriculture and Forestry Sciences, No. 12 Ruiwangfen, Xiangshan, Haidian District, Beijing 100093, PR China c Beijing Vegetable Research Center, Beijing Academy of Agriculture and Forestry Sciences, Banjin, Haidian District, Beijing 100097, PR China a r t i c l e i n f o a b s t r a c t Article history: Esters are a very important component of strawberry (Fragaria sp.) aroma. In this study, fruit volatiles Received 8 April 2012 were isolated by solid-phase microextraction and analyzed by gas chromatography–mass spectrometry Received in revised form 1 November 2012 (SPME/GC–MS). It was found that F. × ananassa had more esters than F. vesca, but there was no significant Accepted 1 November 2012 difference in average relative content of esters. Twenty five esters were selected as predominant esters, 16 of which were prevalent in F. × ananassa and another 16 in F. vesca. Among them, hexyl acetate, Keywords: octyl acetate, ethyl butyrate, ethyl hexanoate, ethyl octanoate, methyl decanoate, and ethyl decanoate Volatile ester composition were found in both F. -

N-Propyl Acetate Technical Data Sheet
Technical Data Sheet Product Name n-Propyl Acetate Synonyms Acetic Acid, n-propyl Ester Chemical Formula CH3COOC3H7 Product Description N-propyl acetate is a colorless, volatile solvent with an odor similar to acetone. It has good solvency power for many natural and synthetic resins. It is miscible with many organic solvents. Applications • Coatings • Wood lacquers • Aerosol sprays • Nail care • Cosmetic / personal care solvent • Fragrance solvent • Process solvent • Printing inks (especially flexographic and special screen) Typical Physical Properties Property Value Molecular Weight (g/mol) 102.13 Boiling Point @ 760 mmHg, 1.01 ar 101.5 °C (214.7 °F) Flash Point (Setaflash Closed Cup) 11.8 °C (53.24°F) Freezing Point -93 °C (-135.4°F) Vapor pressure@ 25°C — extrapolated 25 mmHg 4.79 Kpa Specific gravity (20/20°C) 0.888 Liquid Density @ 20°C 0.89 g/cm3 Vapor Density (air = 1) 3.5 Viscosity (cP or mPa•s @ 20°C) 0.6 Surface tension (dynes/cm or mN/m @ 20°C) 24.4 Specific heat (J/g/°C @ 25°C) No test data available Heat of vaporization (J/g) at normal boiling No test data available point Net heat of combustion (kJ/g) — predicted @ No test data available 25°C Autoignition temperature 380 °C (716 °F) Evaporation rate (n-butyl acetate = 1.0) 2.75 Solubility, g/L or % @ 20°C Solvent in water 2% Water in solvent 2.6% Hansen solubility parameters (J/cm³)1/2 _Total 8.6 _Non-Polar 7.5 _Polar 2.1 Form No. 327-00024-0812 Page 1 of 3 ®™Trademark of The Dow Chemical Company (“Dow”) or an affiliated company of Dow _Hydrogen bonding 3.7 Partition Coefficient, n-octanol/water 1.4 (log Pow) Flammable limits (vol.% in air) Lower 1.7 Upper 8.0 Typical Physical Properties: This data provided for those properties are typical values, and should not be construed as sales specifications.