A Mass Discreditation of Gqn Minerals
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mineral Processing
Mineral Processing Foundations of theory and practice of minerallurgy 1st English edition JAN DRZYMALA, C. Eng., Ph.D., D.Sc. Member of the Polish Mineral Processing Society Wroclaw University of Technology 2007 Translation: J. Drzymala, A. Swatek Reviewer: A. Luszczkiewicz Published as supplied by the author ©Copyright by Jan Drzymala, Wroclaw 2007 Computer typesetting: Danuta Szyszka Cover design: Danuta Szyszka Cover photo: Sebastian Bożek Oficyna Wydawnicza Politechniki Wrocławskiej Wybrzeze Wyspianskiego 27 50-370 Wroclaw Any part of this publication can be used in any form by any means provided that the usage is acknowledged by the citation: Drzymala, J., Mineral Processing, Foundations of theory and practice of minerallurgy, Oficyna Wydawnicza PWr., 2007, www.ig.pwr.wroc.pl/minproc ISBN 978-83-7493-362-9 Contents Introduction ....................................................................................................................9 Part I Introduction to mineral processing .....................................................................13 1. From the Big Bang to mineral processing................................................................14 1.1. The formation of matter ...................................................................................14 1.2. Elementary particles.........................................................................................16 1.3. Molecules .........................................................................................................18 1.4. Solids................................................................................................................19 -

Thermal Annealing and Phase Transformation of Serpentine-Like Garnierite
minerals Article Thermal Annealing and Phase Transformation of Serpentine-Like Garnierite Arun Kumar 1,2 , Michele Cassetta 1, Marco Giarola 3, Marco Zanatta 4 , Monique Le Guen 5, Gian Domenico Soraru 6 and Gino Mariotto 1,* 1 Department of Computer Science, University of Verona, 37134 Verona, Italy; [email protected] (A.K.); [email protected] (M.C.) 2 CNR-Institute for Microelectronics and Microsystems, Agrate Brianza, 20864 Agrate, Italy 3 Centro Piattaforme Tecnologiche (CPT), University of Verona, 37134 Verona, Italy; [email protected] 4 Department of Physics, University of Trento, 38123 Povo, Italy; [email protected] 5 Innovation Technology Direction, ERAMET IDEAS, 78190 Trappes, France; [email protected] 6 Department of Industrial Engineering, University of Trento, 38123 Povo, Italy; [email protected] * Correspondence: [email protected] Abstract: This study is focused on the vibrational and microstructural aspects of the thermally induced transformation of serpentine-like garnierite into quartz, forsterite, and enstatite occur- ring at about 620 ◦C. Powder specimens of garnierite were annealed in static air between room temperature and 1000 ◦C. The kinetic of the transformation was investigated by means of thermo- gravimetric and differential thermal analysis, and the final product was extensively characterized via micro-Raman spectroscopy and X-ray diffraction. Our study shows that serpentine-like garnierite consists of a mixture of different mineral species. Furthermore, these garnierites and their compo- sition can provide details based on the mineralogy and the crystalline phases resulting from the thermal treatment. Citation: Kumar, A.; Cassetta, M.; Giarola, M.; Zanatta, M.; Le Guen, M.; Keywords: garnierite; phase transformation; TGA/DSC; XRD; micro-Raman spectroscopy Soraru, G.D.; Mariotto, G. -

DOGAMI MP-20, Investigations of Nickel in Oregon
0 C\1 a: w a.. <( a.. en ::::> 0 w z <( __j __j w () en � INVESTIGATIONS OF NICKEL IN OREGON STATE OF OREGON DEPARTMENT OF GEOL.OGY AND MINERAL. IN OUSTRIES DONAL.D .A HUL.L. STATE GEOLOGIST 1978 STATE OF OREGON DEPARTMENT OF GEOLOGY AND MINERAL INDUSTRIES 1069 State Office Building, Portland, Oregon 97201 MISCELLANEOUS PAPER 20 INVESTIGATIONS OF NICKEL IN OREGON Len Ramp, Resident Geologist Grants Pass Field Office Oregon Department of Geology and Mineral Industries Conducted in conformance with ORS 516.030 . •. 5 1978 GOVERNING BOARD Leeanne MacColl, Chairperson, Portland Talent Robert W. Doty STATE GEOLOGIST John Schwabe Portland Donald A. Hull CONTENTS INTRODUCTION -- - ---- -- -- --- Purpose and Scope of this Report Acknowledgments U.S. Nickel Industry GEOLOGY OF LATERITE DEPOSITS - -- - 3 Previous Work - - - - --- 3 Ultramafic Rocks - ----- --- 3 Composition - - -------- - 3 Distribution ------ - - - 3 Structure - 3 Geochemistry of Nickel ---- 4 Chemical Weathering of Peridotite - - 4 The soi I profile ------- 5 M i nero I ogy -- - ----- 5 Prospecting Guides and Techniques- - 6 OTHER TYPES OF NICKEL DEPOSITS - - 7 Nickel Sulfide Deposits- - - - - - 7 Deposits in Oregon 7 Other areas --- 8 Prospecting techniques 8 Silica-Carbonate Deposits - -- 8 DISTRIBUTION OF LATERITE DEPOSITS - ------ 9 Nickel Mountain Deposits - - ------ --------- 9 Location --------------- --- 9 Geology - ------- ----- 11 Ore deposits ----------- - -- 11 Soil mineralogy - ------- 12 Structure --- ---- ---- 13 Mining and metallurgy ------------ ---- 13 Production- -
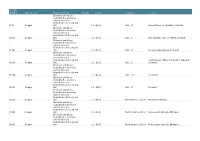
BRSUG Number Mineral Name Hey Index Group Hey No
BRSUG Number Mineral name Hey Index Group Hey No. Chem. Country Locality Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-37 Copper Au) 1.1 4[Cu] U.K., 17 Basset Mines, nr. Redruth, Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-151 Copper Au) 1.1 4[Cu] U.K., 17 Phoenix mine, Cheese Wring, Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-280 Copper Au) 1.1 4[Cu] U.K., 17 County Bridge Quarry, Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and South Caradon Mine, 4 miles N of Liskeard, B-319 Copper Au) 1.1 4[Cu] U.K., 17 Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-394 Copper Au) 1.1 4[Cu] U.K., 17 ? Cornwall? Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-395 Copper Au) 1.1 4[Cu] U.K., 17 Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-539 Copper Au) 1.1 4[Cu] North America, U.S.A Houghton, Michigan Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-540 Copper Au) 1.1 4[Cu] North America, U.S.A Keweenaw Peninsula, Michigan, Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-541 Copper Au) 1.1 4[Cu] North America, U.S.A Keweenaw Peninsula, Michigan, Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, -

New Mineral Names*,†
American Mineralogist, Volume 106, pages 1360–1364, 2021 New Mineral Names*,† Dmitriy I. Belakovskiy1, and Yulia Uvarova2 1Fersman Mineralogical Museum, Russian Academy of Sciences, Leninskiy Prospekt 18 korp. 2, Moscow 119071, Russia 2CSIRO Mineral Resources, ARRC, 26 Dick Perry Avenue, Kensington, Western Australia 6151, Australia In this issue This New Mineral Names has entries for 11 new species, including 7 minerals of jahnsite group: jahnsite- (NaMnMg), jahnsite-(NaMnMn), jahnsite-(CaMnZn), jahnsite-(MnMnFe), jahnsite-(MnMnMg), jahnsite- (MnMnZn), and whiteite-(MnMnMg); lasnierite, manganflurlite (with a new data for flurlite), tewite, and wumuite. Lasnierite* the LA-ICP-MS analysis, but their concentrations were below detec- B. Rondeau, B. Devouard, D. Jacob, P. Roussel, N. Stephant, C. Boulet, tion limits. The empirical formula is (Ca0.59Sr0.37)Ʃ0.96(Mg1.42Fe0.54)Ʃ1.96 V. Mollé, M. Corre, E. Fritsch, C. Ferraris, and G.C. Parodi (2019) Al0.87(P2.99Si0.01)Ʃ3.00(O11.41F0.59)Ʃ12 based on 12 (O+F) pfu. The strongest lines of the calculated powder X-ray diffraction pattern are [dcalc Å (I%calc; Lasnierite, (Ca,Sr)(Mg,Fe)2Al(PO4)3, a new phosphate accompany- ing lazulite from Mt. Ibity, Madagascar: an example of structural hkl)]: 4.421 (83; 040), 3.802 (63, 131), 3.706 (100; 022), 3.305 (99; 141), characterization from dynamic refinement of precession electron 2.890 (90; 211), 2.781 (69; 221), 2.772 (67; 061), 2.601 (97; 023). It diffraction data on submicrometer sample. European Journal of was not possible to perform powder nor single-crystal X-ray diffraction Mineralogy, 31(2), 379–388. -

Characterisation of Ni Silicate-Bearing Minerals by UV-Vis-NIR Spectrscopy
This may be the author’s version of a work that was submitted/accepted for publication in the following source: Bayyareddy, Jagannadha, Frost, Ray,& Dickfos, Marilla (2009) Characterisation of Ni silicate-bearing minerals by UV-vis-NIR spec- trscopy: Effect of Ni substitution in hydrous Ni-Mg silicates. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 71(5), pp. 1762-1768. This file was downloaded from: https://eprints.qut.edu.au/15661/ c Copyright 2009 Elsevier Reproduced in accordance with the copyright policy of the publisher. Notice: Please note that this document may not be the Version of Record (i.e. published version) of the work. Author manuscript versions (as Sub- mitted for peer review or as Accepted for publication after peer review) can be identified by an absence of publisher branding and/or typeset appear- ance. If there is any doubt, please refer to the published source. https://doi.org/10.1016/j.saa.2008.06.030 QUT Digital Repository: http://eprints.qut.edu.au/ Reddy, B. Jagannadha and Frost, Ray L. and Dickfos, Marilla J. (2009) Characterisation of Ni silicate-bearing minerals by UV-Vis-NIR spectroscopy - Effect of Ni substitution in hydrous Ni-Mg silicates . Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 71:pp. 1762-1768. © Copyright 2009 Elsevier 1 2 Characterisation of Ni silicate-bearing minerals by UV-Vis-NIR spectroscopy 3 -Effect of Ni substitution in hydrous Ni-Mg silicates 4 5 B. Jagannadha Reddy, Ray L. Frost •and Marilla J. Dickfos 6 7 Inorganic Materials Research program, School of Physical and Chemical Sciences, 8 Queensland University of Technology, GPO Box 2434, Brisbane Queensland 4001, 9 Australia. -

The Phase Diversity of Nickel Phyllosilicates from New Caledonia: an Investigation Using Transmission Electron Microscopy (TEM) STUDENT: Brittany A
THE PHASE DIVERSITY OF NICKEL PHYLLOSILICATES FROM NEW CALEDONIA: AN INVESTIGATION USING TRANSMISSION ELECTRON MICROSCOPY (TEM) A THESIS SUBMITTED TO THE GRADUATE SCHOOL IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE MASTER OF SCIENCE BY BRITTANY CYMES DR. KIRSTEN NICHOLSON – ADVISOR BALL STATE UNIVERSITY MUNCIE, INDIANA MAY 2016 ABSTRACT THESIS: The Phase Diversity of Nickel Phyllosilicates from New Caledonia: An Investigation Using Transmission Electron Microscopy (TEM) STUDENT: Brittany A. Cymes DEGREE: Master of Science COLLEGE: Sciences and Humanities DATE: May, 2016 PAGES: 136 New Caledonia is a French territorial island in the Southwest Pacific with an economy heavily dependent upon nickel-mining, being the 5th largest producer worldwide. The nickel deposits result from tropical lateritic weathering of ophiolite units that were emplaced during the late Eocene. The ultramafic units weathered and continue to weather into bright green Ni-rich phyllosilicates colloquially referred to as „garnierite‟. Detailed investigations of „garnierite‟ using transmission electron microscopy (TEM) have been carried out in other locations to characterize important nanoscale features; however, none have been undertaken in New Caledonia. In this investigation, ten samples of „garnierite‟ from New Caledonia were examined with powder X-ray diffraction (XRD) and TEM with energy dispersive spectrometry (EDS). The XRD results show the phyllosilicate material to be comprised of highly disordered talc- and serpentine-like minerals with minor chlorite indicated by significantly broad reflections and two- i dimensional diffraction bands, consistent with published data. TEM analysis, however, revealed new information. Talc-like minerals occur as fluffy aggregates of crystals with lattices with approximately 10 Å d-spacing and significant crystallographic disorder and also as aggregates of larger crystals with more orderly lattices; these two varieties represent the kerolite-pimelite series and the talc- willemseite series, respectively. -

Talc- and Serpentine-Like “Garnierites” from Falcondo Ni
macla nº 15 . septiembre 2011 revista de la sociedad española de mineralogía 197 Talc- and Serpentine-like “Garnierites” from Falcondo Ni-laterite Deposit (Dominican Republic): a HRTEM approach / CRISTINA VILLANOVA-DE-BENAVENT (1,*), FERNANDO NIETO (2), JOAQUÍN A. PROENZA (1), SALVADOR GALÍ (1) (1) Departament de Cristal·lografia, Mineralogia i Dipòsits Minerals. Facultat de Geologia. Universitat de Barcelona. C/ Martí i Franquès s/n. 08028 Barcelona, Catalunya (España) (2) Departamento de Mineralogía y Petrología e Instituto Andaluz de Ciencias de la Tierra. Universidad de Granada-CSIC. Campus de • Fuerteventura s/n. 18002 Granada (España) INTRODUCTION. from Falcondo Ni-laterite deposit. These “garnierites” mainly occur as mm-cm results are compared to those previously vein fillings in fractures, but also as “Garnierites” represent significant Ni ore obtained through powder XRD and EMP coatings, boxworks and different kinds minerals in the lower horizons of many (CCiT, Universitat de Barcelona). of breccias, within the saprolite horizon Ni-laterite deposits worldwide (e.g. or near the unweathered peridotite, Freyssinet et al., 2005). They consist of Preliminary data allows to classify the close to the base of the lateritic profile. a green, fine-grained mixture of hydrous sampled “garnierites” into two groups, Ni-bearing magnesium phyllosilicates, which display two well distinguishable METHODOLOGY. including serpentine, talc, sepiolite, greenish colours in hand specimen: smectite and chlorite (e.g. Brindley and A representative sample, containing Hang, 1973; Springer, 1974; Brindley et • Bluish bright-green “garnierites” strongly serpentinized peridotite al., 1979). Thus, “garnierite” is a general display colloform textures under the (saprolite) cross-cut by talc-like and descriptive term and is not recognized optical microscope. -

The Journal of R
The Journal of r Volumemmoie 28 Noemmoi. emmoi2 Apriol 200 2 o Notes from the Laboratory Chocolate- brown Opal Inclutsfbns in Gemstones Natural Amethyst hi1 (iemmoiotiuMl Asstvi.it inn .nij C iem Tcstiiv* I .ahoivitoi'v oi Crrot Britain Gemmological Association and Gem Testing Laboratory of Great Britain 27 Greville Street, London ECIN 8TN Tel: 020 7404 3334 Fax: 020 7404 8843 e-mail: [email protected] Website: www.gagtl.ac.uk President: Professor AT. Collins Vice-Presidents: N. W. Deeks, AE. Farn, RA Howie, D.G. Kent, RK. Mitchell Honorary Fellows: Chen Zhonghui, RA Howie, RT. Liddicoat [nr, K. Nassau Honorary Life Members: H. Bank, D.J. Callaghan, E.A [obbins, H. Tillander Council of Management: T.J. Davidson, RR Harding, 1. Mercer, J. Monnickendam, M.I. O'Donoghue, E. Stern, 1. Thomson, v.P. Watson Members' Council: AJ. Allnutt, S. Burgoyne, P. Dwyer-Hickey, S.A. Everitt, J. Greatwood, B. Jackson, L. Music, J.B. Nelson, P.G. Read, P.J. Wates, C.H. Winter Branch Chairmen: Midlands - G.M. Green, North West - D. M. Brady, Scottish - B. Jackson, South West - RM. Slater Examiners: AJ. Allnutt, M.Sc., PhD., FGA, L. Bartlett, B.5c., M.Phil., FGA, DGA, S. Coelho, B.5c., FGA, DGA, Prof. AT. Collins, B.Sc., Ph.D, A.G. Good, FGA, DGA, I. Greatwood, FGA, G.M. Howe, FGA, DGA, S. Hue Williams MA, FGA, DGA, B. Jackson, FGA, DGA, G.B. Jones, B.5c., Ph.D., FGA, Li Li Ping, FGA, DGA, M. Newton, B.Sc.,D.Phil., c.I.E. Oldershaw, B.Sc. -

The Picking Table Volume 8, No. 1
THE PICKING TABLE FRANKLIN OGDENSBURG MINSHALOGICAL SOCIETY, INC, P. 0. BOJC 146 FRANKLIN, H.J., 07416 VOLUME VIII FEBRUARY 196? NUMBER 1 The contents of The Picking Table are licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. CLUB PROGRAM - SPRING 1967 All meetings will be held at the Hardyston School, intersection of Routes #23 and #517, Franklin, N. J. Pre meeting activities start at 1:00 P.A. Speaker will be announced at 2:30 P.M. Sunday, Field trip, 9=00 A..-I. to Noon - March 19th. Buckwheat Dump, Franklin, N.J. Meeting, 2:30 P.M. Speaker, Paul Desautels Subject - Blood Relatives Among the Minerals. Saturday, Field trip, 9:00 A.M. to Noon - April 15th Buckwheat Dump, Franklin, N.J. Meeting, 2:30 P.M. Speaker - Dr. Paul Moore. Subject - The Mineralogy of Langban, Sweden. Saturday, Field trip, 9:00 A.M. to Noon - Open Cuts, May 20th Sterling Hill Mine, Ogdensburg, H. J. Meeting, 2:30 P.M. Speaker - Dr. Clifford Frondel Subject - Franklin Minerals, New and Old Saturday, Field trio, 9:00 A.M. to Noon - June 17th Farber Quarry, Cork Hill Road, Franklin, ..J, Meeting, 2:30 P.JL Speaker - Robert Metsger Subject - The Geology of Sterling Hill. Special Events April 22/23 1967 Earth Science and Gem Show Mineralogical Society of Pennsylvania, Route 30, Lancaster, Pa. May 6/7th 3rd Annual Mineral and Gem Show Matawan Mineralogical Society, Matawan Regional High School, Matawan, ft. June 29/July 2nd 1967 National Gem and Mineral Show, Eastern Federation, Washington Hilton Hotel, Washington, D.C. -

(12) United States Patent (10) Patent No.: US 8,318,652 B2 Fernandes Et Al
USOO83 18652B2 (12) United States Patent (10) Patent No.: US 8,318,652 B2 Fernandes et al. (45) Date of Patent: Nov. 27, 2012 (54) COLORED SPECKLES COMPRISINGA 4.488.972 A 12/1984 Weinstein ...................... 252.86 POROUS CARRIER AND A RELEASING g s A : 5. 3. REal. ..................... $64. AGENT LAYER 6,281,187WW - B1 * 8/2001 SmerZnakCal. ....... ... 510,418 6,384,008 B1* 5/2002 Parry .......... ... 510,414 (75) Inventors: Gregory Fernandes, Greenville, SC 6,436,889 B1 8/2002 Sta et al. ................ ... 510,298 (US); Eduardo Torres, Spartanburg, SC 6,541,437 B2 4/2003 Mendez Mata et al. ...... 510,348 (US) 7.002051 B2 * 2/2006 Bonelli et al. ......... ... 510,301 7,033,983 B2 * 4/2006 Beers et al. ............ ... 510,344 O O 2001.0053757 A1* 12/2001 Mendez Mata et al. ...... 510,499 (73) Assignee: Milliken & Company, Spartanburg, SC 2003/0087791 A1* 5, 2003 Bonelli et al. ......... ... 510,444 (US) 2003/0162680 A1 8/2003 Sta et al. ............ ... 510,298 2003/0166489 A1* 9, 2003 Van Asten et al. ... 510,440 (*) Notice: Subject to any disclaimer, the term of this 2004/0018955 A1 1/2004 Wevers et al. .................. 512/27 patent is extended or adjusted under 35 2005/0148486 A1* 7/2005 Schramm et al. ............. 510,419 2006/0111264 A1 5, 2006 Smets et al. ....... ... 510/475 U.S.C. 154(b) by 0 days. 2010/0125957 A1* 5/2010 Hong et al. ... ... 8,524 (21) Appl. No.: 12/851,616 2010, 0132134 A1* 6, 2010 Smets et al. ...................... 8,441 ppl. No.: 9 FOREIGN PATENT DOCUMENTS (22) Filed: Aug. -

Supplementary Material Impact of Ferrous Iron Dosing on Iron and Phosphorus Solids Speciation and Transformation in a Pilot Scal
Electronic Supplementary Material (ESI) for Environmental Science: Water Research & Technology. This journal is © The Royal Society of Chemistry 2019 Supplementary Material Impact of ferrous iron dosing on iron and phosphorus solids speciation and transformation in a pilot scale membrane bioreactor Hao Wu1, Yuan Wang1, Atsushi Ikeda-Ohno2, Christopher J. Miller1 and T. David Waite1,* 1 Water Research Centre, School of Civil & Environmental Engineering, The University of New South Wales, Sydney 2052, Australia 2 Collaborative Laboratories for Advanced Decommissioning Science (CLADS), Japan Atomic Energy Agency (JAEA), 2-4 Shirakata, Tokai-mura, Naka-gun, Ibaraki 319-1195, Japan Environmental Science: Water Research & Technology *Corresponding author: Professor T. David Waite, Email: [email protected] 1 X-ray absorption spectroscopy (XAS) Data collection Iron speciation was studied by X-ray absorption spectroscopy including both X-ray absorption near-edge structure (XANES) and extended X-ray absorption fine structure (EXAFS) regions at the Fe K-edge (7112.0 eV). The X-ray absorption spectra were collected on beamline 17C1 at the National Synchrotron Radiation Research Center (NSRRC), Taiwan (Tsang et al. 1995) using a Si(111) double-crystal monochromator with a Rh-coated mirror. Samples were ground to fine powder, pressed into a pellet of 5 mm diameter and 1.0 to 2.5 mm in thickness, and sealed in plastic bags. For iron reference compounds and some sludge samples with high iron concentrations, a small amount of the sample was mixed with boron nitride to dilute the sample to achieve an appropriate edge jump at the Fe K-edge. The X-ray energy of the acquired spectra was calibrated by the simultaneous measurement of the Fe K- edge for Fe metal foil (defined as 7112.0 eV at the first inflection point).