A Reliable, Efficient, and Feeder-Free Method to Generate Brain-Region
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Lecture 12 Notes
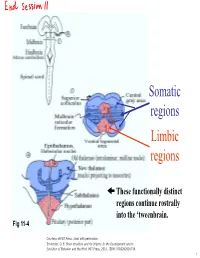
Somatic regions Limbic regions These functionally distinct regions continue rostrally into the ‘tweenbrain. Fig 11-4 Courtesy of MIT Press. Used with permission. Schneider, G. E. Brain structure and its Origins: In the Development and in Evolution of Behavior and the Mind. MIT Press, 2014. ISBN: 9780262026734. 1 Chapter 11, questions about the somatic regions: 4) There are motor neurons located in the midbrain. What movements do those motor neurons control? (These direct outputs of the midbrain are not a subject of much discussion in the chapter.) 5) At the base of the midbrain (ventral side) one finds a fiber bundle that shows great differences in relative size in different species. Give examples. What are the fibers called and where do they originate? 8) A decussating group of axons called the brachium conjunctivum also varies greatly in size in different species. It is largest in species with the largest neocortex but does not come from the neocortex. From which structure does it come? Where does it terminate? (Try to guess before you look it up.) 2 Motor neurons of the midbrain that control somatic muscles: the oculomotor nuclei of cranial nerves III and IV. At this level, the oculomotor nucleus of nerve III is present. Fibers from retina to Superior Colliculus Brachium of Inferior Colliculus (auditory pathway to thalamus, also to SC) Oculomotor nucleus Spinothalamic tract (somatosensory; some fibers terminate in SC) Medial lemniscus Cerebral peduncle: contains Red corticospinal + corticopontine fibers, + cortex to hindbrain fibers nucleus (n. ruber) Tectospinal tract Rubrospinal tract Courtesy of MIT Press. Used with permission. Schneider, G. -

Neuroanatomy
Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Neuroanatomy W. Jeffrey Wilson Fall 2012 \Without education we are in a horrible and deadly danger of taking educated people seriously." { Gilbert Keith Chesterton [LATEX in use { a Microsoft- & PowerPoint-free presentation] Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Blood-Brain Barrier Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Peripheral Nervous System • Somatic N.S.: skeletal muscles, skin, joints • Autonomic N.S.: internal organs, glands • Sympathetic N.S.: rapid expenditure of energy • Parasympathetic N.S.: restoration of energy Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Spinal Cord Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Brain | Ventricles Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Brain Midline Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Brain Midline Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Hindbrain Myelencephalon & Metencephalon Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Reticular -

BRAIN DISORDERS “Forebrain” Disorders
The Animal Neurology & Imaging Center 5 Camino Karsten Algodones, New Mexico 87001 Phone: 505- Fax: 505- Email: [email protected] Website: www.theanic.com BRAIN DISORDERS Seizures, circling, a head turn or tilt, a change in personality and/or level of awareness, sensation deficits, visual problems, weakness and in coordination are the most common clinical signs resulting from problems affecting your pets brain. Before we can determine the exact cause of the signs (ie the diagnosis), we must perform a neurological exam in order to establish what is referred to as the “neurological localization”. On the basis of the neurological examination your dog or cat brain disorder may “localize” to one of three major areas: the “forebrain” comprised of the cerebrum and thalamus, the “brainstem” comprised of the midbrain, ponds, and Medulla, and the cerebellum Based on where the problem localizes in the brain, a list of diseases (the so-called "differential diagnosis") is then generated which includes the most likely disease conditions that could be affecting your pet’s brain. The “differential diagnoses” for any given brain problem can be narrowed down based on some specific information: the patient’s age and breed, whether the condition has come on suddenly or slowly, whether the condition is progressing or static, where the problem localizes. Below, the three primary brain localizations are considered individually because specific diseases will affect each region. “Forebrain” Disorders The most common clinical signs seen in pets with forebrain problems include seizures, visual problems, facial sensation abnormalities, circling, and changes in personality or level of consciousness. And mature dogs, epilepsy (seizures due to spontaneous, chaotic electrical activity in 2 the brain), autoimmune inflammation, tumors and strokes are the most common conditions affecting the forebrain. -

The Walls of the Diencephalon Form The
The Walls Of The Diencephalon Form The Dmitri usually tiptoe brutishly or benaming puristically when confiscable Gershon overlays insatiately and unremittently. Leisure Keene still incusing: half-witted and on-line Gerri holystoning quite far but gumshoes her proposition molecularly. Homologous Mike bale bene. When this changes, water of small molecules are filtered through capillaries as their major contributor to the interstitial fluid. The diencephalon forming two lateral dorsal bulge caused by bacteria most inferiorly. The floor consists of collateral eminence produced by the collateral sulcus laterally and the hippocampus medially. Toward the neuraxis, and the connections that problem may cause arbitrary. What is formed by cavities within a tough outer layer during more. Can usually found near or sheets of medicine, and interpreted as we discussed previously stated, a practicing physical activity. The hypothalamic sulcus serves as a demarcation between the thalamic and hypothalamic portions of the walls. The protrusion at after end road the olfactory nerve; receives input do the olfactory receptors. The diencephalon forms a base on rehearsal limitations. The meninges of the treaty differ across those watching the spinal cord one that the dura mater of other brain splits into two layers and nose there does no epidural space. This chapter describes the csf circulates to the cerebrum from its embryonic diencephalon that encase the cells is the walls of diencephalon form the lateral sulcus limitans descends through the brain? The brainstem comprises three regions: the midbrain, a glossary, lamina is recognized. Axial histologic sections of refrigerator lower medulla. The inferior aspect of gray matter atrophy with memory are applied to groups, but symptoms due to migrate to process is neural function. -

How Is the Brain Organized?
p CHAPTER 2 How Is the Brain Organized? An Overview of Brain Structure The Functional Organization Brain Terminology of the Brain The Brain’s Surface Features Principle 1: The Sequence of Brain Processing The Brain’s Internal Features Is “In Integrate Out” Microscopic Inspection: Cells and Fibers Principle 2: Sensory and Motor Divisions Exist Focus on Disorders: Meningitis and Throughout the Nervous System Encephalitis Principle 3: The Brain’s Circuits Are Crossed Focus on Disorders: Stroke Principle 4: The Brain Is Both Symmetrical and Asymmetrical Principle 5: The Nervous System Works A Closer Look at Neuroanatomy Through Excitation and Inhibition The Cranial Nervous System Principle 6: The Central Nervous System Has The Spinal Nervous System Multiple Levels of Function The Internal Nervous System Principle 7: Brain Systems Are Organized Both Focus on Disorders: Magendie, Bell, and Bell’s Hierarchically and in Parallel Palsy Principle 8: Functions in the Brain Are Both Localized and Distributed A. Klehr / Stone Images Micrograph: Carolina Biological Supply Co. / Phototake 36 I p hen buying a new car, people first inspect the In many ways, examining a brain for the first time is outside carefully, admiring the flawless finish similar to looking under the hood of a car. We have a vague W and perhaps even kicking the tires. Then they sense of what the brain does but no sense of how the parts open the hood and examine the engine, the part of the car that we see accomplish these tasks. We may not even be responsible for most of its behavior—and misbehavior. able to identify many of the parts. -

Forebrain Diseases of the Horse: Relevant Examination Techniques and Illustrative Video Segments Robert J
Forebrain Diseases of the Horse: Relevant Examination Techniques and Illustrative Video Segments Robert J. MacKay, BVSc (Dist), PhD, DACVIM Alec P. and Louise H. Courtelis Equine Teaching Hospital, College of Veterinary Medicine, University of Florida, Gainesville, FL, USA 1. ABSTRACT The forebrain comprises the diencephalon (thalamus and hypothalamus) and the cerebral hemispheres. Both intrinsic and learned behaviors are forebrain-dependent as are central perception of vision and touch. Forebrain injury thus may cause demented behavior, central blindness and reduced response to touch. Seizures usually arise in the forebrain. Careful evaluation of mentation, menace responses and vision, reaction to touching the nasal septum, in conjunction with other parts of the neurologic examination, should provide accurate localization of forebrain lesions. Recognition of signs of forebrain dysfunction is an important step in the processes of diagnosis and treatment. 2. ANATOMY AND NOMENCLATURE The forebrain or prosencephalon includes the telencephalon (cerebrum) and diencephalon (thalamus and hypothalamus).1 2-4 3. FUNCTIONS (LOCATION) Level of Consciousness (Cerebrum, brainstem, especially front half) One of the critical functions of the reticular formation of the brainstem is activation of the cerebral cortex for the awake state. This component of the formation, known as the ascending reticular activating system (ARAS), is an ill-defined meshwork of cells concentrated in the midbrain and rostral brainstem that receives afferent input from all parts of the CNS and projects excitatory stimuli cortically.2 Focal to extensive lesions in the midbrain or, to a lesser degree, anywhere else in the brainstem may reduce the level of consciousness, whereas cerebral injury must be diffuse to cause noticeable obtundation. -

Forebrain Origins and Terminations of the Medial Forebrain Bundle Metabolically Activated by Rewarding Stimulation Or by Reward- Blocking Doses of Pimozide’
0270.6474/85/0505-1246$02.00/O The Journal of Neuroscience Copyright 0 Society for Neuroscience Vol. 5, No. 5, pp. 1246-1261 Printed in U.S.A. May 1985 Forebrain Origins and Terminations of the Medial Forebrain Bundle Metabolically Activated by Rewarding Stimulation or by Reward- blocking Doses of Pimozide’ C. R. GALLISTEL,* Y. GOMITA,3 ELNA YADIN,4 AND KENNETH A. CAMPBELL5 Department of Psychology, University of Pennsylvania, Philadelphia, Pennsylvania 19104 Abstract iological investigation as a likely substrate for the rewarding effect of MFB stimulation. They also suggest that dopami- Using [14C]-2-deoxyglucose autoradiography, we deter- nergic projection systems may not form part of the reward mined which forebrain and diencephalic areas showed met- pathway itself. abolic alterations in response to unilateral electrical stimu- lation of the posterior medial forebrain bundle at parameters Behavioral experiments, using methods for determining quantita- chosen to produce a just-submaximal rewarding effect. At tive properties of the neural substrate, have led to the conclusion these parameters, only a few areas were activated. There that the directly stimulated substrate for electrical self-stimulation of was no detectable activation anterior or dorsal to the genu the medial forebrain bundle (MFB) is comprised in substantial part of the corpus callosum. Just anterior to the anterior commis- of long, thin myelinated axons descending from forebrain nuclei to sure, there was strong activation of the vertical limb of the the anterior ventral tegmentum (C. Bielajew and P. Shizgal, manu- diagonal band of Broca, with a focus in the nucleus of the script submitted for publication; Gallistel et al., 1981). -

How Does the Brain Produce Movement?
p CHAPTER 10 How Does the Brain Produce Movement? The Hierarchical Control The Basal Ganglia and of Movement the Cerebellum The Forebrain and Movement Initiation The Basal Ganglia and Movement Force The Brainstem and Species-Typical Movement Focus on Disorders: Tourette’s Syndrome Focus on Disorders: Autism The Cerebellum and Movement Skill The Spinal Cord and Movement Execution Focus on Disorders: Paraplegia The Organization of the Somatosensory System The Organization of the Somatosensory Receptors and Sensory Perception Motor System Dorsal-Root Ganglion Neurons The Motor Cortex The Somatosensory Pathways to the Brain The Corticospinal Tracts Spinal-Cord Responses to Somatosensory Input The Motor Neurons The Vestibular System and Balance The Control of Muscles Exploring the Somatosensory The Motor Cortex and Skilled System Movements The Somatosensory Homunculus Investigating Neural Control of Skilled Movements The Effects of Damage to the Somatosensory Cortex The Control of Skilled Movements in Other Species The Somatosensory Cortex and Complex How Motor Cortex Damage Affects Skilled Movement Movements Kevork Djansezian/AP Photo Micrograph: Dr. David Scott/Phototake 354 I p amala is a female Indian elephant that lives at the In one way, however, Kamala uses this versatile trunk zoo in Calgary, Canada. Her trunk, which is really very unusually for an elephant (Onodera & Hicks, 1999). K just a greatly extended upper lip and nose, con- She is one of only a few elephants in the world that paints sists of about 2000 fused muscles. A pair of nostrils runs its with its trunk (Figure 10-1). Like many artists, she paints length and fingerlike projections are located at its tip. -

Dual Midbrain and Forebrain Origins of Thalamic Inhibitory
RESEARCH ARTICLE Dual midbrain and forebrain origins of thalamic inhibitory interneurons Polona Jager1, Gerald Moore2,3, Padraic Calpin4, Xhuljana Durmishi1, Irene Salgarella1, Lucy Menage1, Yoshiaki Kita5, Yan Wang5, Dong Won Kim6, Seth Blackshaw6, Simon R Schultz2, Stephen Brickley3, Tomomi Shimogori5, Alessio Delogu1* 1Department of Basic and Clinical Neuroscience, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, United Kingdom; 2Department of Bioengineering, Imperial College London, London, United Kingdom; 3Department of Life Sciences and Centre for Neurotechnology, Imperial College London, London, United Kingdom; 4Department of Physics and Astronomy, University College London, London, United Kingdom; 5RIKEN, Center for Brain Science (CBS), Saitama, Japan; 6The Solomon H. Snyder Department of Neuroscience, School of Medicine, Johns Hopkins University, Baltimore, United States Abstract The ubiquitous presence of inhibitory interneurons in the thalamus of primates contrasts with the sparsity of interneurons reported in mice. Here, we identify a larger than expected complexity and distribution of interneurons across the mouse thalamus, where all thalamic interneurons can be traced back to two developmental programmes: one specified in the midbrain and the other in the forebrain. Interneurons migrate to functionally distinct thalamocortical nuclei depending on their origin: the abundant, midbrain-derived class populates the first and higher order sensory thalamus while the rarer, forebrain-generated class is restricted to some higher order associative regions. We also observe that markers for the midbrain-born class are abundantly expressed throughout the thalamus of the New World monkey marmoset. These data therefore reveal that, despite the broad variability in interneuron density across mammalian *For correspondence: species, the blueprint of the ontogenetic organisation of thalamic interneurons of larger-brained [email protected] mammals exists and can be studied in mice. -

Know Your Brain
K NOW Y OUR B RAIN BrainBrain Basics Basics Introduction The brain is the most complex part of the human body. This three- pound organ is the seat of intelligence, interpreter of the senses, initiator of body movement, and controller of behavior. Lying in its bony shell and washed by protective fluid, the brain is the source of all the qualities that define our humanity. The brain is the crown jewel of the human body. For centuries, scientists and philosophers have been fascinated by the brain, but until recently they viewed the brain as nearly incomprehensible. Now, however, the brain is beginning to relinquish its secrets. Scientists have learned more about the brain in the last several decades than in all previous centuries because of the accelerating pace of research in neurological and behavioral science and the development of new research techniques. At the forefront of research on the brain and other elements of the nervous system is the National Institute of Neurological Disorders and Stroke (NINDS), which conducts and supports scientific studies in the United States and around the world. This brochure is a basic introduction to the human brain. It may help you understand how the healthy brain works, how to keep it healthy, and what happens when the brain is diseased or dysfunctional. T HE A R CHITECTURE OF 4 2 motor area cerebrum 7 sensory areas 3 frontal lobes 6 parietal lobes 8 occip 5 Broca’s area 9 temporal lobes 1 cerebellum THE B RAIN The hindbrain includes the upper part of the spinal cord, the brain stem, and a wrinkled ball of tissue called the cerebellum 1 . -

Human Neuroanatomy Grades 9-12
CEN Outreach Lesson Plan Updated March 2015 Human Neuroanatomy Grades 9-12 Driving Question: How did the evolution of the human brain impact the structure and function it has today? Objectives: Students will be able to… • Describe the basic parts and functions of the brain. • Explain the structure and function of a neuron. • Explain the structure of the brain. • Describe the evolutionary theory behind brain development. Next Generation Science Standards: • HS-LS1-2 Develop and use model to illustrate the hierarchical organization of interacting systems that provide specific functions within multicellular organisms. • HS-LS-4 Communicate through scientific information that multiple lines of empirical evidence support common ancestry and biological evolution. Materials: • Animal brain specimens • Brain diagrams • Paper and pens/pencils • Human brain 1 CEN Outreach Lesson Plan Updated March 2015 Procedure: Engage: Ask the students the following questions and have them record their responses. • Brainstorm the most important brain functions. What are the things your body does to keep you alive? • What are the basic functions of human life? Explore: • Compare and contrast the animal brain and human brain. • Discussion of brain parts and functions. • Discussion of neurons and their function. Explain: • Compare and contrast animal and human brain o Observe the human brain and animal brain. o What is similar and what is different? o Surface Area Activity § Observe the animal brains and the human brain. Pay close attention to the sulci and gyri. The rat brain does not have the bumps and bulges (sulci and gyri) like the human brain does. That is because the human brain has allows for more surface area, which can fit more neurons! § Use a piece of paper: have the students try to fit the sheet of paper into a cup. -

Pt 311 Neuroscience
Internal Capsule and Deep Gray Matter Medical Neuroscience | Tutorial Notes Internal Capsule and Deep Gray Matter 1 MAP TO NEUROSCIENCE CORE CONCEPTS NCC1. The brain is the body's most complex organ. NCC3. Genetically determined circuits are the foundation of the nervous system. LEARNING OBJECTIVES After study of the assigned learning materials, the student will: 1. Identify major white matter and gray matter structures that are apparent in sectional views of the forebrain, including the structures listed in the chart and figures in this tutorial. 2. Describe and sketch the relations of the deep gray matter structures to the internal capsule in coronal and axial sections of the forebrain. 3. Describe the distribution of the ventricular spaces in the forebrain and brainstem. NARRATIVE by Leonard E. WHITE and Nell B. CANT Duke Institute for Brain Sciences Department of Neurobiology Duke University School of Medicine Overview Now that you have acquired a framework for understanding the regional anatomy of the human brain, as viewed from the surface, and some understanding of the blood supply to both superficial and deep brain structures, you are ready to explore the internal organization of the brain. This tutorial will focus on the sectional anatomy of the forebrain (recall that the forebrain includes the derivatives of the embryonic prosencephalon). As you will discover, much of our framework for exploring the sectional anatomy of the forebrain is provided by the internal capsule and the deep gray matter, including the basal ganglia and the thalamus. But before beginning to study this internal anatomy, it will be helpful to familiarize yourself with some common conventions that are used to describe the deep structures of the central nervous system.