Reversal of Hyperactive Subthalamic Circuits Differentially Mitigates Pain Hypersensitivity Phenotypes in Parkinsonian Mice
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Circuits in the Rodent Brainstem That Control Whisking in Concert with Other Orofacial Motor Actions
Neuroscience 368 (2018) 152–170 CIRCUITS IN THE RODENT BRAINSTEM THAT CONTROL WHISKING IN CONCERT WITH OTHER OROFACIAL MOTOR ACTIONS y y LAUREN E. MCELVAIN, a BETH FRIEDMAN, a provides the reset to the relevant premotor oscillators. HARVEY J. KARTEN, b KAREL SVOBODA, c FAN WANG, d Third, direct feedback from somatosensory trigeminal e a,f,g MARTIN DESCHEˆ NES AND DAVID KLEINFELD * nuclei can rapidly alter motion of the sensors. This feed- a Department of Physics, University of California at San Diego, back is disynaptic and can be tuned by high-level inputs. La Jolla, CA 92093, USA A holistic model for the coordination of orofacial motor actions into behaviors will encompass feedback pathways b Department of Neurosciences, University of California at San Diego School of Medicine, La Jolla, CA 92093, USA through the midbrain and forebrain, as well as hindbrain c areas. Howard Hughes Medical Institute, Janelia Research This article is part of a Special Issue entitled: Barrel Campus, Ashburn, VA 20147, USA Cortex. Ó 2017 IBRO. Published by Elsevier Ltd. All rights d Department of Neurobiology, Duke University Medical reserved. Center, Durham, NC 27710, USA e Department of Psychiatry and Neuroscience, Laval University, Que´bec City, G1J 2G3, Canada f Key words: coupled oscillators, facial nucleus, hypoglossal Section of Neurobiology, University of California at San Diego, La Jolla, CA 92093, USA nucleus, licking, orienting, tongue, vibrissa. g Department of Electrical and Computer Engineering, University of California at San Diego, La Jolla, CA 92093, USA Contents Introduction 153 Abstract—The world view of rodents is largely determined Coordination of multiple orofacial motor actions 153 by sensation on two length scales. -

Electrophysiological and Morphological Evidence for a Gabaergic Nigrostriatal Pathway
The Journal of Neuroscience, June 1, 1999, 19(11):4682–4694 Electrophysiological and Morphological Evidence for a GABAergic Nigrostriatal Pathway Manuel Rodrı´guez1 and Toma´ s Gonza´ lez-Herna´ ndez2 Departments of 1Physiology and 2Anatomy, Faculty of Medicine, University of La Laguna, La Laguna, Tenerife, Canary Islands, Spain The electrophysiological and neurochemical characteristics of gic neurons, a percentage that reached 81–84% after 6-OHDA the nondopaminergic nigrostriatal (NO-DA) cells and their func- injection. Electrophysiologically, NO-DA cells showed a behavior tional response to the degeneration of dopaminergic nigrostri- similar to that found in other nigral GABAergic (nigrothalamic) atal (DA) cells were studied. Three different criteria were used to cells. In addition, the 6-OHDA degeneration of DA cells induced a identify NO-DA cells: (1) antidromic response to striatal stimu- modification of their electrophysiological pattern similar to that lation with an electrophysiological behavior (firing rate, inter- found in GABAergic nigrothalamic neurons. Taken together, the spike interval variability, and conduction velocity) different from present data indicate the existence of a small GABAergic nigro- that of DA cells; (2) retrograde labeling after striatal injection of striatal pathway and suggest their involvement in the pathophys- HRP but showing immunonegativity for DA cell markers (ty- iology of Parkinson’s disease. rosine hydroxylase, calretinin, calbindin-D28k, and cholecysto- kinin); and (3) resistance to neurotoxic effect of 6-hydroxydomine Key words: nigrostriatal pathway; dopaminergic cells; (6-OHDA). Our results showed that under normal conditions, GABAergic cells; GABA; glutamic acid decarboxylase; parval- 5–8% of nigrostriatal neurons are immunoreactive for GABA, glu- bumin; calretinin; calbindin-D28k; cholecystokinin; Parkinson’s tamic acid decarboxylase, and parvalbumin, markers of GABAer- disease The substantia nigra (SN) is a major output center of the basal paminergic (Grace and Bunney, 1980, 1983a). -

The Effects of Chloride Dynamics on Substantia Nigra Pars Reticulata
RESEARCH ARTICLE The effects of chloride dynamics on substantia nigra pars reticulata responses to pallidal and striatal inputs Ryan S Phillips1,2, Ian Rosner2,3, Aryn H Gittis2,3, Jonathan E Rubin1,2* 1Department of Mathematics, University of Pittsburgh, Pittsburgh, United States; 2Center for the Neural Basis of Cognition, Pittsburgh, United States; 3Department of Biological Sciences, Carnegie Mellon University, Pittsburgh, United States Abstract As a rodent basal ganglia (BG) output nucleus, the substantia nigra pars reticulata (SNr) is well positioned to impact behavior. SNr neurons receive GABAergic inputs from the striatum (direct pathway) and globus pallidus (GPe, indirect pathway). Dominant theories of action selection rely on these pathways’ inhibitory actions. Yet, experimental results on SNr responses to these inputs are limited and include excitatory effects. Our study combines experimental and computational work to characterize, explain, and make predictions about these pathways. We observe diverse SNr responses to stimulation of SNr-projecting striatal and GPe neurons, including biphasic and excitatory effects, which our modeling shows can be explained by intracellular chloride processing. Our work predicts that ongoing GPe activity could tune the SNr operating mode, including its responses in decision-making scenarios, and GPe output may modulate synchrony and low-frequency oscillations of SNr neurons, which we confirm using optogenetic stimulation of GPe terminals within the SNr. Introduction The substantia nigra pars reticulata (SNr) is the primary output nucleus of the rodent basal ganglia *For correspondence: (BG) and hence likely plays a key role in the behavioral functions, such as decision-making and action [email protected] selection, suppression, or tuning, to which the BG contribute. -
Lecture 12 Notes
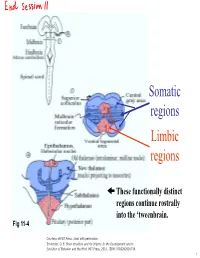
Somatic regions Limbic regions These functionally distinct regions continue rostrally into the ‘tweenbrain. Fig 11-4 Courtesy of MIT Press. Used with permission. Schneider, G. E. Brain structure and its Origins: In the Development and in Evolution of Behavior and the Mind. MIT Press, 2014. ISBN: 9780262026734. 1 Chapter 11, questions about the somatic regions: 4) There are motor neurons located in the midbrain. What movements do those motor neurons control? (These direct outputs of the midbrain are not a subject of much discussion in the chapter.) 5) At the base of the midbrain (ventral side) one finds a fiber bundle that shows great differences in relative size in different species. Give examples. What are the fibers called and where do they originate? 8) A decussating group of axons called the brachium conjunctivum also varies greatly in size in different species. It is largest in species with the largest neocortex but does not come from the neocortex. From which structure does it come? Where does it terminate? (Try to guess before you look it up.) 2 Motor neurons of the midbrain that control somatic muscles: the oculomotor nuclei of cranial nerves III and IV. At this level, the oculomotor nucleus of nerve III is present. Fibers from retina to Superior Colliculus Brachium of Inferior Colliculus (auditory pathway to thalamus, also to SC) Oculomotor nucleus Spinothalamic tract (somatosensory; some fibers terminate in SC) Medial lemniscus Cerebral peduncle: contains Red corticospinal + corticopontine fibers, + cortex to hindbrain fibers nucleus (n. ruber) Tectospinal tract Rubrospinal tract Courtesy of MIT Press. Used with permission. Schneider, G. -

Distribution of Dopamine D3 Receptor Expressing Neurons in the Human Forebrain: Comparison with D2 Receptor Expressing Neurons Eugenia V
Distribution of Dopamine D3 Receptor Expressing Neurons in the Human Forebrain: Comparison with D2 Receptor Expressing Neurons Eugenia V. Gurevich, Ph.D., and Jeffrey N. Joyce, Ph.D. The dopamine D2 and D3 receptors are members of the D2 important difference from the rat is that D3 receptors were subfamily that includes the D2, D3 and D4 receptor. In the virtually absent in the ventral tegmental area. D3 receptor rat, the D3 receptor exhibits a distribution restricted to and D3 mRNA positive neurons were observed in sensory, mesolimbic regions with little overlap with the D2 receptor. hormonal, and association regions such as the nucleus Receptor binding and nonisotopic in situ hybridization basalis, anteroventral, mediodorsal, and geniculate nuclei of were used to study the distribution of the D3 receptors and the thalamus, mammillary nuclei, the basolateral, neurons positive for D3 mRNA in comparison to the D2 basomedial, and cortical nuclei of the amygdala. As revealed receptor/mRNA in subcortical regions of the human brain. by simultaneous labeling for D3 and D2 mRNA, D3 mRNA D2 binding sites were detected in all brain areas studied, was often expressed in D2 mRNA positive neurons. with the highest concentration found in the striatum Neurons that solely expressed D2 mRNA were numerous followed by the nucleus accumbens, external segment of the and regionally widespread, whereas only occasional D3- globus pallidus, substantia nigra and ventral tegmental positive-D2-negative cells were observed. The regions of area, medial preoptic area and tuberomammillary nucleus relatively higher expression of the D3 receptor and its of the hypothalamus. In most areas the presence of D2 mRNA appeared linked through functional circuits, but receptor sites coincided with the presence of neurons co-expression of D2 and D3 mRNA suggests a functional positive for its mRNA. -

Using High-Resolution MR Imaging at 7T to Evaluate the Anatomy of the Midbrain ORIGINAL RESEARCH Dopaminergic System
Using High-Resolution MR Imaging at 7T to Evaluate the Anatomy of the Midbrain ORIGINAL RESEARCH Dopaminergic System M. Eapen BACKGROUND AND PURPOSE: Dysfunction of DA neurotransmission from the SN and VTA has been D.H. Zald implicated in neuropsychiatric diseases, including Parkinson disease and schizophrenia. Unfortunately, these midbrain DA structures are difficult to define on clinical MR imaging. To more precisely evaluate J.C. Gatenby the anatomic architecture of the DA midbrain, we scanned healthy participants with a 7T MR imaging Z. Ding system. Here we contrast the performance of high-resolution T2- and T2*-weighted GRASE and FFE J.C. Gore MR imaging scans at 7T. MATERIALS AND METHODS: Ten healthy participants were scanned by using GRASE and FFE se- quences. CNRs were calculated among the SN, VTA, and RN, and their volumes were estimated by using a segmentation algorithm. RESULTS: Both GRASE and FFE scans revealed visible contrast between midbrain DA regions. The GRASE scan showed higher CNRs compared with the FFE scan. The T2* contrast of the FFE scan further delineated substructures and microvasculature within the midbrain SN and RN. Segmentation and volume estimation of the midbrain SN, RN, and VTA showed individual differences in the size and volume of these structures across participants. CONCLUSIONS: Both GRASE and FFE provide sufficient CNR to evaluate the anatomy of the midbrain DA system. The FFE in particular reveals vascular details and substructure information within the midbrain regions that could be useful for examining -

III. Biochemical and Developmental Dissociation of Patch-Matrix Mesostriatal Systems
The Journal of Neuroscience, December 1987. 7(12): 39353944 The Neostriatal Mosaic: III. Biochemical and Developmental Dissociation of Patch-Matrix Mesostriatal Systems Charles R. Gerfen,’ Ken G. Baimbridge,2 and Jean Thibault3 ‘Laboratory of Neurophysiology and Laboratory of Cell Biology, National Institute of Mental Health, Bethesda, Maryland 20892, ‘Department of Physiology, University of British Columbia, Vancouver, BC, Canada, and 3Department of Cellular Biochemistry, Collkge de France, Paris In the previous paper (Gerfen et al., 1987) mesostriatal do- ically distinct. For example, following pharmacologic treat- paminergic neurons were shown to be subdivided into dorsal ments that deplete dopamine stores in mesostriatal terminals, and ventral tiers that project to the striatal matrix and patch there is a more rapid replenishment of dopamine in patch than compartments, respectively. The present study provides ex- in matrix areas (Olson et al., 1972; Fuxe et al., 1979; Fukui et perimental evidence that these patch-matrix mesostriatal al., 1986). Also, chronic apomorphine treatment results in an dopaminergic systems are biochemically and develop- increase in dynorphin immunoreactivity in patch and not matrix mentally distinct. A 28 kDa calcium-binding protein (CaBP, striatonigral neurons (Li et al., 1986). The manner in which the or calbindin-D 28LDa) is expressed in dorsal tier mesostriatal patch- and matrix-directed mesostriatal dopaminergic neurons dopaminergic neurons. The distribution of such neurons, lo- may be differentially regulated by the compartmental organi- cated in the ventral tegmental area, dorsal tier of the sub- zation of striatonigral projections (Gerfen, 1984, 1985) was dis- stantia nigra pars compacta, and retrorubral area, matches cussed in the previous paper (Gerfen et al., 1987). -

Neuroanatomy
Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Neuroanatomy W. Jeffrey Wilson Fall 2012 \Without education we are in a horrible and deadly danger of taking educated people seriously." { Gilbert Keith Chesterton [LATEX in use { a Microsoft- & PowerPoint-free presentation] Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Blood-Brain Barrier Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Peripheral Nervous System • Somatic N.S.: skeletal muscles, skin, joints • Autonomic N.S.: internal organs, glands • Sympathetic N.S.: rapid expenditure of energy • Parasympathetic N.S.: restoration of energy Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Spinal Cord Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Brain | Ventricles Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Brain Midline Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Brain Midline Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Hindbrain Myelencephalon & Metencephalon Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Reticular -

Descending Projections from the Substantia Nigra Pars Reticulata Differentially Control Seizures
Descending projections from the substantia nigra pars reticulata differentially control seizures Evan Wickera, Veronica C. Becka, Colin Kulick-Sopera, Catherine V. Kulick-Sopera, Safwan K. Hydera, Carolina Campos-Rodrigueza, Tahiyana Khana,b, Prosper N’Gouemoa,b,c, and Patrick A. Forcellia,b,d,1 aDepartment of Pharmacology & Physiology, Georgetown University, Washington, DC 20007; bInterdisciplinary Program in Neuroscience, Georgetown University, Washington, DC 20007; cDepartment of Pediatrics, Georgetown University, Washington, DC 20007; and dDepartment of Neuroscience, Georgetown University, Washington, DC 20007 Edited by Peter L. Strick, University of Pittsburgh, Pittsburgh, PA, and approved November 20, 2019 (received for review May 13, 2019) Three decades of studies have shown that inhibition of the sub- Here, we sought to address 2 unresolved questions. First, to what stantia nigra pars reticulata (SNpr) attenuates seizures, yet the extent does selective silencing of the nigrotectal as compared to the circuits mediating this effect remain obscure. SNpr projects to nigrotegmental pathway recapitulate the effect of silencing SNpr? the deep and intermediate layers of the superior colliculus (DLSC) Second, are the seizure-suppressive effects of SNpr silencing me- and the pedunculopontine nucleus (PPN), but the contributions of diated by divergent pathways depending on the seizure type? these projections are unknown. To address this gap, we optoge- We optogenetically silenced either SNpr, nigrotectal, or nigro- netically silenced cell -

BRAIN DISORDERS “Forebrain” Disorders
The Animal Neurology & Imaging Center 5 Camino Karsten Algodones, New Mexico 87001 Phone: 505- Fax: 505- Email: [email protected] Website: www.theanic.com BRAIN DISORDERS Seizures, circling, a head turn or tilt, a change in personality and/or level of awareness, sensation deficits, visual problems, weakness and in coordination are the most common clinical signs resulting from problems affecting your pets brain. Before we can determine the exact cause of the signs (ie the diagnosis), we must perform a neurological exam in order to establish what is referred to as the “neurological localization”. On the basis of the neurological examination your dog or cat brain disorder may “localize” to one of three major areas: the “forebrain” comprised of the cerebrum and thalamus, the “brainstem” comprised of the midbrain, ponds, and Medulla, and the cerebellum Based on where the problem localizes in the brain, a list of diseases (the so-called "differential diagnosis") is then generated which includes the most likely disease conditions that could be affecting your pet’s brain. The “differential diagnoses” for any given brain problem can be narrowed down based on some specific information: the patient’s age and breed, whether the condition has come on suddenly or slowly, whether the condition is progressing or static, where the problem localizes. Below, the three primary brain localizations are considered individually because specific diseases will affect each region. “Forebrain” Disorders The most common clinical signs seen in pets with forebrain problems include seizures, visual problems, facial sensation abnormalities, circling, and changes in personality or level of consciousness. And mature dogs, epilepsy (seizures due to spontaneous, chaotic electrical activity in 2 the brain), autoimmune inflammation, tumors and strokes are the most common conditions affecting the forebrain. -
![The Effects of Lesions to the Superior Colliculus and Ventromedial Thalamus on [Kappa]-Opioid-Mediated Locomotor Activity in the Preweanling Rat](https://docslib.b-cdn.net/cover/4731/the-effects-of-lesions-to-the-superior-colliculus-and-ventromedial-thalamus-on-kappa-opioid-mediated-locomotor-activity-in-the-preweanling-rat-1924731.webp)
The Effects of Lesions to the Superior Colliculus and Ventromedial Thalamus on [Kappa]-Opioid-Mediated Locomotor Activity in the Preweanling Rat
California State University, San Bernardino CSUSB ScholarWorks Theses Digitization Project John M. Pfau Library 2003 The effects of lesions to the superior colliculus and ventromedial thalamus on [kappa]-opioid-mediated locomotor activity in the preweanling rat Arturo Rubin Zavala Follow this and additional works at: https://scholarworks.lib.csusb.edu/etd-project Part of the Biological Psychology Commons Recommended Citation Zavala, Arturo Rubin, "The effects of lesions to the superior colliculus and ventromedial thalamus on [kappa]-opioid-mediated locomotor activity in the preweanling rat" (2003). Theses Digitization Project. 2404. https://scholarworks.lib.csusb.edu/etd-project/2404 This Thesis is brought to you for free and open access by the John M. Pfau Library at CSUSB ScholarWorks. It has been accepted for inclusion in Theses Digitization Project by an authorized administrator of CSUSB ScholarWorks. For more information, please contact [email protected]. THE EFFECTS OF LESIONS TO THE SUPERIOR COLLICULUS AND VENTROMEDIAL THALAMUS ON k-OPIOID-MEDIATED LOCOMOTOR ACTIVITY IN THE PREWEANLING RAT A Thesis Presented to'the Faculty of California State University, San Bernardino In Partial Fulfillment of the Requirements for the Degree Master of Arts i in I Psychology by Arturo Rubin Zavala March 2003 THE EFFECTS OF LESIONS TO THE SUPERIOR COLLICULUS AND VENTROMEDIAL THALAMUS ON K-OPIOID-MEDIATED LOCOMOTOR ACTIVITY IN THE PREWEANLING RAT A Thesis I Presented to,the Faculty of I California State University, San Bernardino by , Arturo Rubin Zavala March 2003 Approved by: zhwl°3 Sanders A. McDougall, Chair, Psychology Date Thompsorij Biology ABSTRACT The purpose of the present study was to determine the I neuronal circuitry responsible for 'K-opioid-mediated locomotion in preweanling rats. -

The Nuclear Pattern of the Nok-Tectal Portions of the Midbrain and Isthmus in the Opossum
THE NUCLEAR PATTERN OF THE NOK-TECTAL PORTIONS OF THE MIDBRAIN AND ISTHMUS IN THE OPOSSUM RUSSELL T. WOODBURNE Department of Anatomy, Uniwersity of Yichigan SIX PLATES (TWELVE FIGURES) INTRODUCTION It is logical that the present series of descriptions of the nuclear pattern of the midbrain tegmentum in mammals should begin with the account of this region in marsupials, since the American opossum presents a simplified and generalized type of mammalian midbrain. The material employed in the present study consists of toluidin blue series, cut in various planes, of the brain of the American opossum, Didelphis virginiana. These preparations are a part of the Huber Neurological Collection of the Department of Anatomy of the University of Michigan. The literature particularly pertinent to specific nuclear de- scriptions will be discussed in connection with such descrip- tions and the general literature dealing with other than marsupial forms is dealt with in other sections of this series of papers and complete reference made in the comprehensive bibliography. There are, however, certain papers of which some mention should be made. The series of papers by Castaldi ('23, '24, '26) gave the basis for the nomenclature and the general pattern of subdivision followed here. Tsai's ('25) account of portions of the marsupial midbrain, although con- cerned primarily with tectal and pretectal areas, gave some aid in orientation. Certain of the pretectal regions were con- sidered in the light of earlier accounts of Chu ( '32) and Bodian ('40). The text of Ariens Kappers, Huber and Crosby ('36) was used for general orientation and comparative information.