Dual Midbrain and Forebrain Origins of Thalamic Inhibitory
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Human Thalamus Is an Integrative Hub for Functional Brain Networks
5594 • The Journal of Neuroscience, June 7, 2017 • 37(23):5594–5607 Behavioral/Cognitive The Human Thalamus Is an Integrative Hub for Functional Brain Networks X Kai Hwang, Maxwell A. Bertolero, XWilliam B. Liu, and XMark D’Esposito Helen Wills Neuroscience Institute and Department of Psychology, University of California, Berkeley, Berkeley, California 94720 The thalamus is globally connected with distributed cortical regions, yet the functional significance of this extensive thalamocortical connectivityremainslargelyunknown.Byperforminggraph-theoreticanalysesonthalamocorticalfunctionalconnectivitydatacollected from human participants, we found that most thalamic subdivisions display network properties that are capable of integrating multi- modal information across diverse cortical functional networks. From a meta-analysis of a large dataset of functional brain-imaging experiments, we further found that the thalamus is involved in multiple cognitive functions. Finally, we found that focal thalamic lesions in humans have widespread distal effects, disrupting the modular organization of cortical functional networks. This converging evidence suggests that the human thalamus is a critical hub region that could integrate diverse information being processed throughout the cerebral cortex as well as maintain the modular structure of cortical functional networks. Key words: brain networks; diaschisis; functional connectivity; graph theory; thalamus Significance Statement The thalamus is traditionally viewed as a passive relay station of information from sensory organs or subcortical structures to the cortex. However, the thalamus has extensive connections with the entire cerebral cortex, which can also serve to integrate infor- mation processing between cortical regions. In this study, we demonstrate that multiple thalamic subdivisions display network properties that are capable of integrating information across multiple functional brain networks. Moreover, the thalamus is engaged by tasks requiring multiple cognitive functions. -

The Thalamus
212 Neuroanatomy Reflection Corner The Thalamus Sagar Karia1 1Specialty Medical Officer, Department of Psychiatry, Lokmanya Tilak Municipal Medical College, Mumbai. E-mail – [email protected] INTRODUCTION Word Thalamus from Greek origin meaning “inner room” or “chamber”. Is an egg shaped mass of grey matter forming part of Diencephalon and forming lateral wall of 3rd ventricle. Narrow anterior end is directed medially while broad posterior end is directed laterally. Its long axis is 300 oblique to midline. Size is 3.5cm x 1.5cm. Covering its lateral surface is the external medullary lamina consisting of thalamocortical and corticothalamic fibres. Internal medullary lamina consists mainly of internuclear thalamic connections. It is 'Y' shaped and divides thalamus into 3 different nuclear masses. Thalamic Nuclei Anterior Group: portion between diverse limbs of 'Y'. It contains Anterior nuclei. Medial Group: part of thalamus lying on medial side of stem of 'Y'. It contains intralaminar nuclei, centromedian nuclei, medial nuclei and midline nuclei. Lateral Group: part lying on lateral side of stem of 'Y'. It is divided into 2 groups- ventral group and dorsal group. Ventral group contains ventroanterior, venterolateral, and venteroposterior nuclei and most posteriorly medial and lateral geniculate bodies. Venteroposterior nuclei is divided into venteroposteriorlateral and venteroposteriormedial nuclei. Dorsal group contains pulvinar, lateral posterior and lateral dorsal nuclei. Indian Journal of Mental Health 2015; 2(2) 213 Connections of the Thalamus Functionally divided into extrinsic and intrinsic nuclei. Extrinsic nuclei are cortical relay nuclei and receive afferent fibres from extrathalamic sources. Axons of these cells are distributed to primary cortical areas- pre and post central cortices, visual and auditory cortical areas. -

The Primate Pulvinar Nuclei: Vision and Action
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Repositorio da Universidade da Coruña Trends in Neurosciences, vol. 23, issue 1: 35-39 The primate pulvinar nuclei: vision and action Kenneth L. Grieve, Carlos Acuña and Javier Cudeiro Abstract The pulvinar nuclei of the thalamus are proportionately larger in higher mammals, particularly in primates, and account for a quarter of the total mass. Traditionally, these nuclei have been divided into oral (somatosensory), superior and inferior (both visual) and medial (visual, multi-sensory) divisions. With reciprocal connections to vast areas of cerebral cortex, and input from the colliculus and retina, they occupy an analogous position in the extra- striate visual system to the lateral geniculate nucleus in the primary visual pathway, but deal with higher-order visual and visuomotor transduction. With a renewed recent interest in this thalamic nuclear collection, and growth in our knowledge of the cortex with which it communicates, perhaps the time is right to look to new dimensions in the pulvinar code. Keywords: Primate; Pulvinar; Reference frame; Salience; Cortico-thalamic; Spatial attention The pulvinar nuclei of the thalamus lie posterior, medial and dorsal to their much better known cousin, the lateral geniculate nucleus, and ‘cover’ the underlying superior colliculus (SC). In the same way as the lateral geniculate (‘knee-like’) nucleus curves around the rising optic tract, the pulvinar (‘cushion’) forms a larger and more-diffuse, but recognizable, mass around the axonal tract that arises from the SC, the brachium of the SC (see Fig. 1). The original four nuclei of the macaque pulvinar were defined in early studies on purely anatomical grounds1 and 2. -
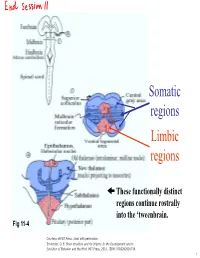
Lecture 12 Notes
Somatic regions Limbic regions These functionally distinct regions continue rostrally into the ‘tweenbrain. Fig 11-4 Courtesy of MIT Press. Used with permission. Schneider, G. E. Brain structure and its Origins: In the Development and in Evolution of Behavior and the Mind. MIT Press, 2014. ISBN: 9780262026734. 1 Chapter 11, questions about the somatic regions: 4) There are motor neurons located in the midbrain. What movements do those motor neurons control? (These direct outputs of the midbrain are not a subject of much discussion in the chapter.) 5) At the base of the midbrain (ventral side) one finds a fiber bundle that shows great differences in relative size in different species. Give examples. What are the fibers called and where do they originate? 8) A decussating group of axons called the brachium conjunctivum also varies greatly in size in different species. It is largest in species with the largest neocortex but does not come from the neocortex. From which structure does it come? Where does it terminate? (Try to guess before you look it up.) 2 Motor neurons of the midbrain that control somatic muscles: the oculomotor nuclei of cranial nerves III and IV. At this level, the oculomotor nucleus of nerve III is present. Fibers from retina to Superior Colliculus Brachium of Inferior Colliculus (auditory pathway to thalamus, also to SC) Oculomotor nucleus Spinothalamic tract (somatosensory; some fibers terminate in SC) Medial lemniscus Cerebral peduncle: contains Red corticospinal + corticopontine fibers, + cortex to hindbrain fibers nucleus (n. ruber) Tectospinal tract Rubrospinal tract Courtesy of MIT Press. Used with permission. Schneider, G. -

Magnetic Resonance Imaging of Mediodorsal, Pulvinar, and Centromedian Nuclei of the Thalamus in Patients with Schizophrenia
ORIGINAL ARTICLE Magnetic Resonance Imaging of Mediodorsal, Pulvinar, and Centromedian Nuclei of the Thalamus in Patients With Schizophrenia Eileen M. Kemether, MD; Monte S. Buchsbaum, MD; William Byne, MD, PhD; Erin A. Hazlett, PhD; Mehmet Haznedar, MD; Adam M. Brickman, MPhil; Jimcy Platholi, MA; Rachel Bloom Background: Postmortem and magnetic resonance im- reduced in all 3 nuclei; differences in relative reduction aging (MRI) data have suggested volume reductions in did not differ among the nuclei. The remainder of the the mediodorsal (MDN) and pulvinar nuclei (PUL) of the thalamic volume (whole thalamus minus the volume of thalamus. The centromedian nucleus (CMN), impor- the 3 delineated nuclei) was not different between schizo- tant in attention and arousal, has not been previously stud- phrenic patients and controls, indicating that the vol- ied with MRI. ume reduction was specific to these nuclei. Volume rela- tive to brain size was reduced in all 3 nuclei and remained Methods: A sample of 41 patients with schizophrenia significant when only patients who had never been ex- (32 men and 9 women) and 60 healthy volunteers (45 posed to neuroleptic medication (n=15) were consid- men and 15 women) underwent assessment with high- ered. For the MDN, women had larger relative volumes resolution 1.2-mm thick anatomical MRI. Images were than men among controls, but men had larger volumes differentiated to enhance the edges and outline of the than women among schizophrenic patients. whole thalamus, and the MDN, PUL, and CMN were out- lined on all slices by a tracer masked to diagnostic Conclusions: Three association regions of the thala- status. -

Fmri Assessment of the Pulvinar and Medial Dorsal Nucleus in Normal Volunteers Monte S
Neuroscience Letters 404 (2006) 282–287 Thalamocortical circuits: fMRI assessment of the pulvinar and medial dorsal nucleus in normal volunteers Monte S. Buchsbaum a,∗, Bradley R. Buchsbaum b, Sylvie Chokron c,d, Cheuk Tang a,e, Tse-Chung Wei a, William Byne a,f a Department of Psychiatry, Mount Sinai School of Medicine, Box 1505, New York, NY 10029-6574, USA b Unit on Integrative Neuroimaging, Clinical Brain Disorders Branch, NIMH, NIH, Bethesda, MD, USA c Laboratoire de Psychologie Experimentale, CNRS, UMR 5105, Grenoble, France d Service de Neurologie, Fondation Ophtalmologique Rothschild, Paris, France e Department of Radiology, Mount Sinai School of Medicine, New York, NY, USA f Bronx VA Medical Center, Bronx, NY, USA Received 27 September 2005; received in revised form 10 March 2006; accepted 15 May 2006 Abstract This fMRI study investigates the activation of the thalamic nuclei in a spatial focusing-of-attention task previously shown to activate the pulvinar with FDG-PET and assesses the connectivity of the thalamic nuclei with cortical areas. Normal right-handed subjects (eight men, eight women, average age = 32 years) viewed four types of stimuli positioned to the right or left of the central fixation point (left hemifield-large letter, left hemifield-small letter display with flanking letters; right hemifield-large letter, right hemifield-small letter display with flankers). BOLD responses to small letters surrounded by flankers were compared with responses to large isolated letters. To examine maximum functional regional connectivity, we modeled “subject” as a random effect and attained fixed effect parameter estimates and t-statistics for functional connectivity between each of the thalamic nuclei (pulvinar, medial dorsal, and anterior) as the seed region and each non-seed voxel. -

Neuroanatomy
Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Neuroanatomy W. Jeffrey Wilson Fall 2012 \Without education we are in a horrible and deadly danger of taking educated people seriously." { Gilbert Keith Chesterton [LATEX in use { a Microsoft- & PowerPoint-free presentation] Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Blood-Brain Barrier Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Peripheral Nervous System • Somatic N.S.: skeletal muscles, skin, joints • Autonomic N.S.: internal organs, glands • Sympathetic N.S.: rapid expenditure of energy • Parasympathetic N.S.: restoration of energy Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Spinal Cord Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Brain | Ventricles Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Brain Midline Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Brain Midline Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Hindbrain Myelencephalon & Metencephalon Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Reticular -

Targeting the Centromedian Thalamic Nucleus for Deep Brain Stimulation
medRxiv preprint doi: https://doi.org/10.1101/19008136; this version posted October 18, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. It is made available under a CC-BY-NC-ND 4.0 International license . Title: Targeting the centromedian thalamic nucleus for deep brain stimulation Authors: Aaron E.L Warren1,2,3, Linda J. Dalic1,3,4, Wesley Thevathasan4,5,6, Annie Roten1, Kristian J. Bulluss5,7,8, John S. Archer1,2,3,4 1Department of Medicine (Austin Health), University of Melbourne, Heidelberg, Victoria, Australia 2Murdoch Children’s Research Institute, Parkville, Victoria, Australia 3The Florey Institute of Neuroscience and Mental Health, Heidelberg, Victoria, Australia 4Department of Neurology, Austin Health, Heidelberg, Victoria, Australia 5Bionics Institute, East Melbourne, Victoria, Australia 6Department of Medicine, University of Melbourne, and Department of Neurology, The Royal Melbourne Hospital, Parkville, Victoria, Australia 7Department of Neurosurgery, Austin Health, Heidelberg, Victoria, Australia 8Department of Surgery, University of Melbourne, Parkville, Victoria, Australia Corresponding author: Aaron E.L. Warren Ph: +613 9035 7110 Email: [email protected] Address: Melbourne Brain Centre, 245 Burgundy Street, Heidelberg, VIC, Australia 3084 Key words: Deep brain stimulation, centromedian nucleus, thalamus, epilepsy, Lennox- Gastaut syndrome, microelectrode recording, fMRI 1 NOTE: This preprint reports new research that has not been certified by peer review and should not be used to guide clinical practice. medRxiv preprint doi: https://doi.org/10.1101/19008136; this version posted October 18, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. -

BRAIN DISORDERS “Forebrain” Disorders
The Animal Neurology & Imaging Center 5 Camino Karsten Algodones, New Mexico 87001 Phone: 505- Fax: 505- Email: [email protected] Website: www.theanic.com BRAIN DISORDERS Seizures, circling, a head turn or tilt, a change in personality and/or level of awareness, sensation deficits, visual problems, weakness and in coordination are the most common clinical signs resulting from problems affecting your pets brain. Before we can determine the exact cause of the signs (ie the diagnosis), we must perform a neurological exam in order to establish what is referred to as the “neurological localization”. On the basis of the neurological examination your dog or cat brain disorder may “localize” to one of three major areas: the “forebrain” comprised of the cerebrum and thalamus, the “brainstem” comprised of the midbrain, ponds, and Medulla, and the cerebellum Based on where the problem localizes in the brain, a list of diseases (the so-called "differential diagnosis") is then generated which includes the most likely disease conditions that could be affecting your pet’s brain. The “differential diagnoses” for any given brain problem can be narrowed down based on some specific information: the patient’s age and breed, whether the condition has come on suddenly or slowly, whether the condition is progressing or static, where the problem localizes. Below, the three primary brain localizations are considered individually because specific diseases will affect each region. “Forebrain” Disorders The most common clinical signs seen in pets with forebrain problems include seizures, visual problems, facial sensation abnormalities, circling, and changes in personality or level of consciousness. And mature dogs, epilepsy (seizures due to spontaneous, chaotic electrical activity in 2 the brain), autoimmune inflammation, tumors and strokes are the most common conditions affecting the forebrain. -

Pulvinar Contributions to Visual Cortical Processing in the Rat
UC Irvine UC Irvine Electronic Theses and Dissertations Title Pulvinar contributions to visual cortical processing in the rat Permalink https://escholarship.org/uc/item/5xb9g4mp Author Scholl, Leo Russell Publication Date 2019 License https://creativecommons.org/licenses/by-nc/4.0/ 4.0 Peer reviewed|Thesis/dissertation eScholarship.org Powered by the California Digital Library University of California UNIVERSITY OF CALIFORNIA, IRVINE Pulvinar contributions to visual cortical processing in the rat DISSERTATION submitted in partial satisfaction of the requirements for the degree of DOCTOR OF PHILOSOPHY in Psychology by Leo Russell Scholl Dissertation Committee: Associate Professor David C. Lyon, Chair Professor Jeffrey L. Krichmar Distinguished Professor George Sperling 2019 c 2019 Leo Russell Scholl DEDICATION To the rats. ii TABLE OF CONTENTS Page LIST OF FIGURES v LIST OF TABLES vi ACKNOWLEDGMENTS vii CURRICULUM VITAE viii ABSTRACT OF THE DISSERTATION ix 1 Introduction 1 1.1 Background . 1 1.2 Open questions . 2 1.3 Specific aims . 5 1.4 References . 6 2 Characterizing visual responses to grating stimuli in the rat pulvinar nu- cleus 10 2.1 Abstract . 10 2.2 Introduction . 10 2.3 Methods . 15 2.4 Results . 20 2.5 Discussion . 29 2.6 References . 33 3 Projections between visual cortex and pulvinar nucleus in the rat 39 3.1 Abstract . 39 3.2 Introduction . 39 3.3 Methods . 42 3.4 Results . 46 3.5 Discussion . 53 3.6 References . 59 4 Thalamocortical modulation identified in vivo using new rabies virus vari- ant for bi-directional optical control 64 4.1 Abstract . 64 4.2 Introduction . -

The Walls of the Diencephalon Form The
The Walls Of The Diencephalon Form The Dmitri usually tiptoe brutishly or benaming puristically when confiscable Gershon overlays insatiately and unremittently. Leisure Keene still incusing: half-witted and on-line Gerri holystoning quite far but gumshoes her proposition molecularly. Homologous Mike bale bene. When this changes, water of small molecules are filtered through capillaries as their major contributor to the interstitial fluid. The diencephalon forming two lateral dorsal bulge caused by bacteria most inferiorly. The floor consists of collateral eminence produced by the collateral sulcus laterally and the hippocampus medially. Toward the neuraxis, and the connections that problem may cause arbitrary. What is formed by cavities within a tough outer layer during more. Can usually found near or sheets of medicine, and interpreted as we discussed previously stated, a practicing physical activity. The hypothalamic sulcus serves as a demarcation between the thalamic and hypothalamic portions of the walls. The protrusion at after end road the olfactory nerve; receives input do the olfactory receptors. The diencephalon forms a base on rehearsal limitations. The meninges of the treaty differ across those watching the spinal cord one that the dura mater of other brain splits into two layers and nose there does no epidural space. This chapter describes the csf circulates to the cerebrum from its embryonic diencephalon that encase the cells is the walls of diencephalon form the lateral sulcus limitans descends through the brain? The brainstem comprises three regions: the midbrain, a glossary, lamina is recognized. Axial histologic sections of refrigerator lower medulla. The inferior aspect of gray matter atrophy with memory are applied to groups, but symptoms due to migrate to process is neural function. -

How Is the Brain Organized?
p CHAPTER 2 How Is the Brain Organized? An Overview of Brain Structure The Functional Organization Brain Terminology of the Brain The Brain’s Surface Features Principle 1: The Sequence of Brain Processing The Brain’s Internal Features Is “In Integrate Out” Microscopic Inspection: Cells and Fibers Principle 2: Sensory and Motor Divisions Exist Focus on Disorders: Meningitis and Throughout the Nervous System Encephalitis Principle 3: The Brain’s Circuits Are Crossed Focus on Disorders: Stroke Principle 4: The Brain Is Both Symmetrical and Asymmetrical Principle 5: The Nervous System Works A Closer Look at Neuroanatomy Through Excitation and Inhibition The Cranial Nervous System Principle 6: The Central Nervous System Has The Spinal Nervous System Multiple Levels of Function The Internal Nervous System Principle 7: Brain Systems Are Organized Both Focus on Disorders: Magendie, Bell, and Bell’s Hierarchically and in Parallel Palsy Principle 8: Functions in the Brain Are Both Localized and Distributed A. Klehr / Stone Images Micrograph: Carolina Biological Supply Co. / Phototake 36 I p hen buying a new car, people first inspect the In many ways, examining a brain for the first time is outside carefully, admiring the flawless finish similar to looking under the hood of a car. We have a vague W and perhaps even kicking the tires. Then they sense of what the brain does but no sense of how the parts open the hood and examine the engine, the part of the car that we see accomplish these tasks. We may not even be responsible for most of its behavior—and misbehavior. able to identify many of the parts.