The Human Thalamus Is an Integrative Hub for Functional Brain Networks
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cortex and Thalamus Lecture.Pptx
Cerebral Cortex and Thalamus Hyperbrain Ch 2 Monica Vetter, PhD January 24, 2013 Learning Objectives: • Anatomy of the lobes of the cortex • Relationship of thalamus to cortex • Layers and connectivity of the cortex • Vascular supply to cortex • Understand the location and function of hypothalamus and pituitary • Anatomy of the basal ganglia • Primary functions of the different lobes/ cortical regions – neurological findings 1 Types of Cortex • Sensory (Primary) • Motor (Primary) • Unimodal association • Multimodal association - necessary for language, reason, plan, imagine, create Note: • Gyri • Sulci • Fissures • Lobes 2 The Thalamus is highly interconnected with the cerebral cortex, and handles most information traveling to or from the cortex. “Specific thalamic Ignore nuclei” – have well- names of defined sensory or thalamic nuclei for motor functions now - A few Other nuclei have will more distributed reappear later function 3 Thalamus Midbrain Pons Limbic lobe = cingulate gyrus Structure of Neocortex (6 layers) white matter gray matter Pyramidal cells 4 Connectivity of neurons in different cortical layers Afferents = inputs Efferents = outputs (reciprocal) brainstem etc Eg. Motor – Eg. Sensory – more efferent more afferent output input Cortico- cortical From Thalamus To spinal cord, brainstem etc. To Thalamus Afferent and efferent connections to different ….Depending on whether they have more layers of cortex afferent or efferent connections 5 Different areas of cortex were defined by differences in layer thickness, and size and -

Basal Ganglia Anatomy, Physiology, and Function Ns201c
Basal Ganglia Anatomy, Physiology, and Function NS201c Human Basal Ganglia Anatomy Basal Ganglia Circuits: The ‘Classical’ Model of Direct and Indirect Pathway Function Motor Cortex Premotor Cortex + Glutamate Striatum GPe GPi/SNr Dopamine + - GABA - Motor Thalamus SNc STN Analagous rodent basal ganglia nuclei Gross anatomy of the striatum: gateway to the basal ganglia rodent Dorsomedial striatum: -Inputs predominantly from mPFC, thalamus, VTA Dorsolateral striatum: -Inputs from sensorimotor cortex, thalamus, SNc Ventral striatum: Striatal subregions: Dorsomedial (caudate) -Inputs from vPFC, hippocampus, amygdala, Dorsolateral (putamen) thalamus, VTA Ventral (nucleus accumbens) Gross anatomy of the striatum: patch and matrix compartments Patch/Striosome: -substance P -mu-opioid receptor Matrix: -ChAT and AChE -somatostatin Microanatomy of the striatum: cell types Projection neurons: MSN: medium spiny neuron (GABA) •striatonigral projecting – ‘direct pathway’ •striatopallidal projecting – ‘indirect pathway’ Interneurons: FS: fast-spiking interneuron (GABA) LTS: low-threshold spiking interneuron (GABA) LA: large aspiny neuron (ACh) 30 um Cellular properties of striatal neurons Microanatomy of the striatum: striatal microcircuits • Feedforward inhibition (mediated by fast-spiking interneurons) • Lateral feedback inhibition (mediated by MSN collaterals) Basal Ganglia Circuits: The ‘Classical’ Model of Direct and Indirect Pathway Function Motor Cortex Premotor Cortex + Glutamate Striatum GPe GPi/SNr Dopamine + - GABA - Motor Thalamus SNc STN The simplified ‘classical’ model of basal ganglia circuit function • Information encoded as firing rate • Basal ganglia circuit is linear and unidirectional • Dopamine exerts opposing effects on direct and indirect pathway MSNs Basal ganglia motor circuit: direct pathway Motor Cortex Premotor Cortex Glutamate Striatum GPe GPi/SNr Dopamine + GABA Motor Thalamus SNc STN Direct pathway MSNs express: D1, M4 receptors, Sub. -

The Thalamus
212 Neuroanatomy Reflection Corner The Thalamus Sagar Karia1 1Specialty Medical Officer, Department of Psychiatry, Lokmanya Tilak Municipal Medical College, Mumbai. E-mail – [email protected] INTRODUCTION Word Thalamus from Greek origin meaning “inner room” or “chamber”. Is an egg shaped mass of grey matter forming part of Diencephalon and forming lateral wall of 3rd ventricle. Narrow anterior end is directed medially while broad posterior end is directed laterally. Its long axis is 300 oblique to midline. Size is 3.5cm x 1.5cm. Covering its lateral surface is the external medullary lamina consisting of thalamocortical and corticothalamic fibres. Internal medullary lamina consists mainly of internuclear thalamic connections. It is 'Y' shaped and divides thalamus into 3 different nuclear masses. Thalamic Nuclei Anterior Group: portion between diverse limbs of 'Y'. It contains Anterior nuclei. Medial Group: part of thalamus lying on medial side of stem of 'Y'. It contains intralaminar nuclei, centromedian nuclei, medial nuclei and midline nuclei. Lateral Group: part lying on lateral side of stem of 'Y'. It is divided into 2 groups- ventral group and dorsal group. Ventral group contains ventroanterior, venterolateral, and venteroposterior nuclei and most posteriorly medial and lateral geniculate bodies. Venteroposterior nuclei is divided into venteroposteriorlateral and venteroposteriormedial nuclei. Dorsal group contains pulvinar, lateral posterior and lateral dorsal nuclei. Indian Journal of Mental Health 2015; 2(2) 213 Connections of the Thalamus Functionally divided into extrinsic and intrinsic nuclei. Extrinsic nuclei are cortical relay nuclei and receive afferent fibres from extrathalamic sources. Axons of these cells are distributed to primary cortical areas- pre and post central cortices, visual and auditory cortical areas. -

The Thalamus: Gateway to the Mind Lawrence M
Overview The thalamus: gateway to the mind Lawrence M. Ward∗ The thalamus of the brain is far more than the simple sensory relay it was long thought to be. From its location at the top of the brain stem it interacts directly with nearly every part of the brain. Its dense loops into and out of cortex render it functionally a seventh cortical layer. Moreover, it receives and sends connections to most subcortical areas as well. Of course it does function as a very sophisticated sensory relay and thus is of vital importance to perception. But also it functions critically in all mental operations, including attention, memory, and consciousness, likely in different ways for different processes, as indicated by the consequences of damage to its various nuclei as well as by invasive studies in nonhuman animals. It plays a critical role also in the arousal system of the brain, in emotion, in movement, and in coordinating cortical computations. Given these important functional roles, and the dearth of knowledge about the details of its nonsensory nuclei, it is an attractive target for intensive study in the future, particularly in regard to its role in healthy and impaired cognitive functioning. © 2013 John Wiley & Sons, Ltd. How to cite this article: WIREs Cogn Sci 2013. doi: 10.1002/wcs.1256 INTRODUCTION and other animals can do quite well without major chunks of cortex. Indeed, decorticate rats behave very pen nearly any textbook of neuroscience or similarly to normal rats in many ways,2 whereas sensation and perception and you will find the O de-thalamate rats die. -

Higher-Order Thalamic Relays Burst More Than First-Order Relays
Higher-order thalamic relays burst more than first-order relays E. J. Ramcharan*, J. W. Gnadt†, and S. M. Sherman‡§ *Center for Complex Systems and Brain Sciences, Florida Atlantic University, 777 Glades Road, Boca Raton, FL 33431; †Department of Neurobiology, State University of New York, Stony Brook, NY 11794-5230; and ‡Department of Neurobiology, Pharmacology, and Physiology, University of Chicago, MC 0926, 316 Abbott, 947 East 58th Street, Chicago, IL 60637 Edited by Robert H. Wurtz, National Institutes of Health, Bethesda, MD, and approved July 11, 2005 (received for review April 6, 2005) There is a strong correlation between the behavior of an animal trasted with the higher-order relays, such as the pulvinar for and the firing mode (burst or tonic) of thalamic relay neurons. vision, the magnocellular (or ‘‘nonlemniscal’’) portion of the Certain differences between first- and higher-order thalamic relays medial geniculate nucleus for hearing, and the posterior medial (which relay peripheral information to the cortex versus informa- nucleus for somesthesis, which are thought to serve as a link in tion from one cortical area to another, respectively) suggest that cortico-thalamo-cortical pathways that continue to process these more bursting might occur in the higher-order relays. Accordingly, information streams. we recorded bursting behavior in single cells from awake, behav- We thought that it would be useful to extend the observations ing rhesus monkeys in first-order (the lateral geniculate nucleus, of bursting to higher-order thalamic relays in the behaving the ventral posterior nucleus, and the ventral portion of the medial monkey for the following reasons. -

The Primate Pulvinar Nuclei: Vision and Action
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Repositorio da Universidade da Coruña Trends in Neurosciences, vol. 23, issue 1: 35-39 The primate pulvinar nuclei: vision and action Kenneth L. Grieve, Carlos Acuña and Javier Cudeiro Abstract The pulvinar nuclei of the thalamus are proportionately larger in higher mammals, particularly in primates, and account for a quarter of the total mass. Traditionally, these nuclei have been divided into oral (somatosensory), superior and inferior (both visual) and medial (visual, multi-sensory) divisions. With reciprocal connections to vast areas of cerebral cortex, and input from the colliculus and retina, they occupy an analogous position in the extra- striate visual system to the lateral geniculate nucleus in the primary visual pathway, but deal with higher-order visual and visuomotor transduction. With a renewed recent interest in this thalamic nuclear collection, and growth in our knowledge of the cortex with which it communicates, perhaps the time is right to look to new dimensions in the pulvinar code. Keywords: Primate; Pulvinar; Reference frame; Salience; Cortico-thalamic; Spatial attention The pulvinar nuclei of the thalamus lie posterior, medial and dorsal to their much better known cousin, the lateral geniculate nucleus, and ‘cover’ the underlying superior colliculus (SC). In the same way as the lateral geniculate (‘knee-like’) nucleus curves around the rising optic tract, the pulvinar (‘cushion’) forms a larger and more-diffuse, but recognizable, mass around the axonal tract that arises from the SC, the brachium of the SC (see Fig. 1). The original four nuclei of the macaque pulvinar were defined in early studies on purely anatomical grounds1 and 2. -

MRI Atlas of the Human Deep Brain Jean-Jacques Lemaire
MRI Atlas of the Human Deep Brain Jean-Jacques Lemaire To cite this version: Jean-Jacques Lemaire. MRI Atlas of the Human Deep Brain. 2019. hal-02116633 HAL Id: hal-02116633 https://hal.uca.fr/hal-02116633 Preprint submitted on 1 May 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution - NonCommercial - NoDerivatives| 4.0 International License MRI ATLAS of the HUMAN DEEP BRAIN Jean-Jacques Lemaire, MD, PhD, neurosurgeon, University Hospital of Clermont-Ferrand, Université Clermont Auvergne, CNRS, SIGMA, France This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/ or send a letter to Creative Commons, PO Box 1866, Mountain View, CA 94042, USA. Terminologia Foundational Model Terminologia MRI Deep Brain Atlas NeuroNames (ID) neuroanatomica usages, classical and french terminologies of Anatomy (ID) Anatomica 1998 (ID) 2017 http://fipat.library.dal.ca In -
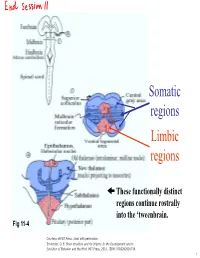
Lecture 12 Notes
Somatic regions Limbic regions These functionally distinct regions continue rostrally into the ‘tweenbrain. Fig 11-4 Courtesy of MIT Press. Used with permission. Schneider, G. E. Brain structure and its Origins: In the Development and in Evolution of Behavior and the Mind. MIT Press, 2014. ISBN: 9780262026734. 1 Chapter 11, questions about the somatic regions: 4) There are motor neurons located in the midbrain. What movements do those motor neurons control? (These direct outputs of the midbrain are not a subject of much discussion in the chapter.) 5) At the base of the midbrain (ventral side) one finds a fiber bundle that shows great differences in relative size in different species. Give examples. What are the fibers called and where do they originate? 8) A decussating group of axons called the brachium conjunctivum also varies greatly in size in different species. It is largest in species with the largest neocortex but does not come from the neocortex. From which structure does it come? Where does it terminate? (Try to guess before you look it up.) 2 Motor neurons of the midbrain that control somatic muscles: the oculomotor nuclei of cranial nerves III and IV. At this level, the oculomotor nucleus of nerve III is present. Fibers from retina to Superior Colliculus Brachium of Inferior Colliculus (auditory pathway to thalamus, also to SC) Oculomotor nucleus Spinothalamic tract (somatosensory; some fibers terminate in SC) Medial lemniscus Cerebral peduncle: contains Red corticospinal + corticopontine fibers, + cortex to hindbrain fibers nucleus (n. ruber) Tectospinal tract Rubrospinal tract Courtesy of MIT Press. Used with permission. Schneider, G. -

Magnetic Resonance Imaging of Mediodorsal, Pulvinar, and Centromedian Nuclei of the Thalamus in Patients with Schizophrenia
ORIGINAL ARTICLE Magnetic Resonance Imaging of Mediodorsal, Pulvinar, and Centromedian Nuclei of the Thalamus in Patients With Schizophrenia Eileen M. Kemether, MD; Monte S. Buchsbaum, MD; William Byne, MD, PhD; Erin A. Hazlett, PhD; Mehmet Haznedar, MD; Adam M. Brickman, MPhil; Jimcy Platholi, MA; Rachel Bloom Background: Postmortem and magnetic resonance im- reduced in all 3 nuclei; differences in relative reduction aging (MRI) data have suggested volume reductions in did not differ among the nuclei. The remainder of the the mediodorsal (MDN) and pulvinar nuclei (PUL) of the thalamic volume (whole thalamus minus the volume of thalamus. The centromedian nucleus (CMN), impor- the 3 delineated nuclei) was not different between schizo- tant in attention and arousal, has not been previously stud- phrenic patients and controls, indicating that the vol- ied with MRI. ume reduction was specific to these nuclei. Volume rela- tive to brain size was reduced in all 3 nuclei and remained Methods: A sample of 41 patients with schizophrenia significant when only patients who had never been ex- (32 men and 9 women) and 60 healthy volunteers (45 posed to neuroleptic medication (n=15) were consid- men and 15 women) underwent assessment with high- ered. For the MDN, women had larger relative volumes resolution 1.2-mm thick anatomical MRI. Images were than men among controls, but men had larger volumes differentiated to enhance the edges and outline of the than women among schizophrenic patients. whole thalamus, and the MDN, PUL, and CMN were out- lined on all slices by a tracer masked to diagnostic Conclusions: Three association regions of the thala- status. -

Learning & Memory
Downloaded from learnmem.cshlp.org on September 27, 2021 - Published by Cold Spring Harbor Laboratory Press Review Unraveling the contributions of the diencephalon to recognition memory: A review John P. Aggleton,1,3 Julie R. Dumont,1 and Elizabeth Clea Warburton2 1School of Psychology, Cardiff University, Cardiff, CF10 3AT, Wales, United Kingdom; 2MRC Centre for Synaptic Plasticity, School of Physiology, University of Bristol, Bristol BS8 1TD, United Kingdom Both clinical investigations and studies with animals reveal nuclei within the diencephalon that are vital for recognition memory (the judgment of prior occurrence). This review seeks to identify these nuclei and to consider why they might be important for recognition memory. Despite the lack of clinical cases with circumscribed pathology within the diencepha- lon and apparent species differences, convergent evidence from a variety of sources implicates a subgroup of medial dien- cephalic nuclei. It is supposed that the key functional interactions of this subgroup of diencephalic nuclei are with the medial temporal lobe, the prefrontal cortex, and with cingulate regions. In addition, some of the clinical evidence most readily supports dual-process models of recognition, which assume two independent cognitive processes (recollective-based and familiarity-based) that combine to direct recognition judgments. From this array of information a “multi-effect multi- nuclei” model is proposed, in which the mammillary bodies and the anterior thalamic nuclei are of preeminent importance for recollective-based recognition. The medial dorsal thalamic nucleus is thought to contribute to familiarity-based recog- nition, but this nucleus, along with various midline and intralaminar thalamic nuclei, is also assumed to have broader, indirect effects upon both recollective-based and familiarity-based recognition. -

Projections of Auditory Cortex to the Medial Geniculate Body of the Cat
THE JOURNAL OF COMPARATIVE NEUROLOGY 430:27–55 (2001) Projections of Auditory Cortex to the Medial Geniculate Body of the Cat JEFFERY A. WINER,* JAMES J. DIEHL, AND DAVID T. LARUE Division of Neurobiology, Department of Molecular and Cell Biology, University of California at Berkeley, Berkeley, California 94720-3200 ABSTRACT The corticofugal projection from 12 auditory cortical fields onto the medial geniculate body was investigated in adult cats by using wheat germ agglutinin conjugated to horserad- ish peroxidase or biotinylated dextran amines. The chief goals were to determine the degree of divergence from single cortical fields, the pattern of convergence from several fields onto a single nucleus, the extent of reciprocal relations between corticothalamic and thalamocortical connections, and to contrast and compare the patterns of auditory corticogeniculate projec- tions with corticofugal input to the inferior colliculus. The main findings were that (1) single areas showed a wide range of divergence, projecting to as few as 5, and to as many as 15, thalamic nuclei; (2) most nuclei received projections from approximately five cortical areas, whereas others were the target of as few as three areas; (3) there was global corticothalamic- thalamocortical reciprocity in every experiment, and there were also significant instances of nonreciprocal projections, with the corticothalamic input often more extensive; (4) the corti- cothalamic projection was far stronger and more divergent than the corticocollicular projec- tion from the same areas, suggesting that the thalamus and the inferior colliculus receive differential degrees of corticofugal control; (5) cochleotopically organized areas had fewer corticothalamic projections than fields in which tonotopy was not a primary feature; and (6) all corticothalamic projections were topographic, focal, and clustered, indicating that areas with limited cochleotopic organization still have some internal spatial arrangement. -

Amygdalar Interaction with the Mediodorsal Nucleus of the Thalamus and the Ventromedial Prefrontal Cortex in Stimulus-Reward Associative Learning in the Monkey
The Journal of Neuroscience, November 1990, fO(11): 3479-3493 Amygdalar Interaction with the Mediodorsal Nucleus of the Thalamus and the Ventromedial Prefrontal Cortex in Stimulus-Reward Associative Learning in the Monkey David Gaffan’ and Elisabeth A. Murray* ‘Department of Experimental Psychology, Oxford University, Oxford OX1 3UD, England, and *Laboratory of Neuropsychology, National Institute of Mental Health, Bethesda, Maryland 20892 Cynomolgus monkeys (Macaca fascicularis) were assessed One kind of associative learning in which the amygdala is of for their ability to associate visual stimuli with food reward. central importance is the associationof a sensorystimulus with They learned a series of new 2-choice visual discriminations the incentive value of food reward. In visual-discrimination between colored patterns displayed on a monitor screen. learning for food reward, the role of the amygdala varies ac- The feedback for correct choice was the delivery of food. In cording to the associative structure of the task. If visual dis- order to promote associative learning between the visual criminative stimuli are associateddirectly with the incentive stimuli and the incentive value of the food reward, reward value of the food reward, efficient learning dependson an in- delivery was not accompanied by any distinctive visual feed- trahemispheric interaction between the amygdala and the vi- back on the display screen. The rate of learning new prob- sual-associationcortex ipsilateral to it (E. A. Gaffan et al., 1988). lems was assessed before and after surgery in a total of 16 But when visual discriminative stimuli are associatedwith the monkeys. Three groups of 3 monkeys received bilaterally incentive value indirectly, via the mediation of a direct asso- symmetrical ablations in either the amygdala, the medio- ciation with an auditory or visual secondary reinforcer, visual dorsal nucleus of the thalamus, or the ventromedial pre- interaction with the amygdala is less important (Gaffan and frontal cortex.