The Thalamus: Gateway to the Mind Lawrence M
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cortex and Thalamus Lecture.Pptx
Cerebral Cortex and Thalamus Hyperbrain Ch 2 Monica Vetter, PhD January 24, 2013 Learning Objectives: • Anatomy of the lobes of the cortex • Relationship of thalamus to cortex • Layers and connectivity of the cortex • Vascular supply to cortex • Understand the location and function of hypothalamus and pituitary • Anatomy of the basal ganglia • Primary functions of the different lobes/ cortical regions – neurological findings 1 Types of Cortex • Sensory (Primary) • Motor (Primary) • Unimodal association • Multimodal association - necessary for language, reason, plan, imagine, create Note: • Gyri • Sulci • Fissures • Lobes 2 The Thalamus is highly interconnected with the cerebral cortex, and handles most information traveling to or from the cortex. “Specific thalamic Ignore nuclei” – have well- names of defined sensory or thalamic nuclei for motor functions now - A few Other nuclei have will more distributed reappear later function 3 Thalamus Midbrain Pons Limbic lobe = cingulate gyrus Structure of Neocortex (6 layers) white matter gray matter Pyramidal cells 4 Connectivity of neurons in different cortical layers Afferents = inputs Efferents = outputs (reciprocal) brainstem etc Eg. Motor – Eg. Sensory – more efferent more afferent output input Cortico- cortical From Thalamus To spinal cord, brainstem etc. To Thalamus Afferent and efferent connections to different ….Depending on whether they have more layers of cortex afferent or efferent connections 5 Different areas of cortex were defined by differences in layer thickness, and size and -

The Human Thalamus Is an Integrative Hub for Functional Brain Networks
5594 • The Journal of Neuroscience, June 7, 2017 • 37(23):5594–5607 Behavioral/Cognitive The Human Thalamus Is an Integrative Hub for Functional Brain Networks X Kai Hwang, Maxwell A. Bertolero, XWilliam B. Liu, and XMark D’Esposito Helen Wills Neuroscience Institute and Department of Psychology, University of California, Berkeley, Berkeley, California 94720 The thalamus is globally connected with distributed cortical regions, yet the functional significance of this extensive thalamocortical connectivityremainslargelyunknown.Byperforminggraph-theoreticanalysesonthalamocorticalfunctionalconnectivitydatacollected from human participants, we found that most thalamic subdivisions display network properties that are capable of integrating multi- modal information across diverse cortical functional networks. From a meta-analysis of a large dataset of functional brain-imaging experiments, we further found that the thalamus is involved in multiple cognitive functions. Finally, we found that focal thalamic lesions in humans have widespread distal effects, disrupting the modular organization of cortical functional networks. This converging evidence suggests that the human thalamus is a critical hub region that could integrate diverse information being processed throughout the cerebral cortex as well as maintain the modular structure of cortical functional networks. Key words: brain networks; diaschisis; functional connectivity; graph theory; thalamus Significance Statement The thalamus is traditionally viewed as a passive relay station of information from sensory organs or subcortical structures to the cortex. However, the thalamus has extensive connections with the entire cerebral cortex, which can also serve to integrate infor- mation processing between cortical regions. In this study, we demonstrate that multiple thalamic subdivisions display network properties that are capable of integrating information across multiple functional brain networks. Moreover, the thalamus is engaged by tasks requiring multiple cognitive functions. -

Basal Ganglia Anatomy, Physiology, and Function Ns201c
Basal Ganglia Anatomy, Physiology, and Function NS201c Human Basal Ganglia Anatomy Basal Ganglia Circuits: The ‘Classical’ Model of Direct and Indirect Pathway Function Motor Cortex Premotor Cortex + Glutamate Striatum GPe GPi/SNr Dopamine + - GABA - Motor Thalamus SNc STN Analagous rodent basal ganglia nuclei Gross anatomy of the striatum: gateway to the basal ganglia rodent Dorsomedial striatum: -Inputs predominantly from mPFC, thalamus, VTA Dorsolateral striatum: -Inputs from sensorimotor cortex, thalamus, SNc Ventral striatum: Striatal subregions: Dorsomedial (caudate) -Inputs from vPFC, hippocampus, amygdala, Dorsolateral (putamen) thalamus, VTA Ventral (nucleus accumbens) Gross anatomy of the striatum: patch and matrix compartments Patch/Striosome: -substance P -mu-opioid receptor Matrix: -ChAT and AChE -somatostatin Microanatomy of the striatum: cell types Projection neurons: MSN: medium spiny neuron (GABA) •striatonigral projecting – ‘direct pathway’ •striatopallidal projecting – ‘indirect pathway’ Interneurons: FS: fast-spiking interneuron (GABA) LTS: low-threshold spiking interneuron (GABA) LA: large aspiny neuron (ACh) 30 um Cellular properties of striatal neurons Microanatomy of the striatum: striatal microcircuits • Feedforward inhibition (mediated by fast-spiking interneurons) • Lateral feedback inhibition (mediated by MSN collaterals) Basal Ganglia Circuits: The ‘Classical’ Model of Direct and Indirect Pathway Function Motor Cortex Premotor Cortex + Glutamate Striatum GPe GPi/SNr Dopamine + - GABA - Motor Thalamus SNc STN The simplified ‘classical’ model of basal ganglia circuit function • Information encoded as firing rate • Basal ganglia circuit is linear and unidirectional • Dopamine exerts opposing effects on direct and indirect pathway MSNs Basal ganglia motor circuit: direct pathway Motor Cortex Premotor Cortex Glutamate Striatum GPe GPi/SNr Dopamine + GABA Motor Thalamus SNc STN Direct pathway MSNs express: D1, M4 receptors, Sub. -
Lecture 12 Notes
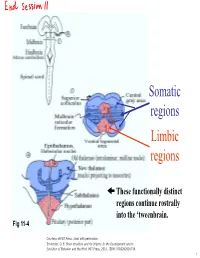
Somatic regions Limbic regions These functionally distinct regions continue rostrally into the ‘tweenbrain. Fig 11-4 Courtesy of MIT Press. Used with permission. Schneider, G. E. Brain structure and its Origins: In the Development and in Evolution of Behavior and the Mind. MIT Press, 2014. ISBN: 9780262026734. 1 Chapter 11, questions about the somatic regions: 4) There are motor neurons located in the midbrain. What movements do those motor neurons control? (These direct outputs of the midbrain are not a subject of much discussion in the chapter.) 5) At the base of the midbrain (ventral side) one finds a fiber bundle that shows great differences in relative size in different species. Give examples. What are the fibers called and where do they originate? 8) A decussating group of axons called the brachium conjunctivum also varies greatly in size in different species. It is largest in species with the largest neocortex but does not come from the neocortex. From which structure does it come? Where does it terminate? (Try to guess before you look it up.) 2 Motor neurons of the midbrain that control somatic muscles: the oculomotor nuclei of cranial nerves III and IV. At this level, the oculomotor nucleus of nerve III is present. Fibers from retina to Superior Colliculus Brachium of Inferior Colliculus (auditory pathway to thalamus, also to SC) Oculomotor nucleus Spinothalamic tract (somatosensory; some fibers terminate in SC) Medial lemniscus Cerebral peduncle: contains Red corticospinal + corticopontine fibers, + cortex to hindbrain fibers nucleus (n. ruber) Tectospinal tract Rubrospinal tract Courtesy of MIT Press. Used with permission. Schneider, G. -

05 Trigeminal System 2013.Pdf
Dental Neuroanatomy Thursday February 7th, 2013 David A. Morton, Ph.D. 5. THE TRIGEMINAL SYSTEM Somatic Sensation of the Face and Head Objectives 1. Outline the two pathways for facial sensation from the head. 2. Contrast facial sensation from the head and somatic sensation from the body. In what ways are they similar? Different? Try drawing this on the Haines atlas diagram at the end of the lecture. 3. Diagram the corneal reflex: the afferent and efferent limbs as well as nuclei involved in the brainstem. 4. If a person does not blink, how would you determine if the problem were in the sensory (afferent) limb, motor (efferent) limb, or brainstem interconnections for the corneal reflex? 5. Explain how a single, small medullary vascular lesion could abolish pain and temperature from the face on the right side and pain and temperature from the body on the left side. What vessel is most likely occluded? Introduction – The trigeminal system for the face and oral cavity is organized in a manner similar to the spinal cord. It has the equivalent of both the DCML pathway and the ALS pathway. The two trigeminal pathways will converge in the thalamus. The most confusing thing is that one of them descends before crossing and the other crosses immediately. Peripheral Receptors and Sensation Structures served by trigeminal system. 1. Cornea 2. Mucocutaneous tissues around mouth and nostrils. 3. Oral and nasal mucosae 4. Paranasal sinuses 5. Tongue (anterior two thirds) 6. Teeth and gums 7. Dura of anterior and middle cranial fossae 8. Skin of face to the vertex except angle of jaw 9. -

BRAIN DISORDERS “Forebrain” Disorders
The Animal Neurology & Imaging Center 5 Camino Karsten Algodones, New Mexico 87001 Phone: 505- Fax: 505- Email: [email protected] Website: www.theanic.com BRAIN DISORDERS Seizures, circling, a head turn or tilt, a change in personality and/or level of awareness, sensation deficits, visual problems, weakness and in coordination are the most common clinical signs resulting from problems affecting your pets brain. Before we can determine the exact cause of the signs (ie the diagnosis), we must perform a neurological exam in order to establish what is referred to as the “neurological localization”. On the basis of the neurological examination your dog or cat brain disorder may “localize” to one of three major areas: the “forebrain” comprised of the cerebrum and thalamus, the “brainstem” comprised of the midbrain, ponds, and Medulla, and the cerebellum Based on where the problem localizes in the brain, a list of diseases (the so-called "differential diagnosis") is then generated which includes the most likely disease conditions that could be affecting your pet’s brain. The “differential diagnoses” for any given brain problem can be narrowed down based on some specific information: the patient’s age and breed, whether the condition has come on suddenly or slowly, whether the condition is progressing or static, where the problem localizes. Below, the three primary brain localizations are considered individually because specific diseases will affect each region. “Forebrain” Disorders The most common clinical signs seen in pets with forebrain problems include seizures, visual problems, facial sensation abnormalities, circling, and changes in personality or level of consciousness. And mature dogs, epilepsy (seizures due to spontaneous, chaotic electrical activity in 2 the brain), autoimmune inflammation, tumors and strokes are the most common conditions affecting the forebrain. -

Thalamus.Pdf
Thalamus 583 THALAMUS This lecture will focus on the thalamus, a subdivision of the diencephalon. The diencephalon can be divided into four areas, which are interposed between the brain stem and cerebral hemispheres. The four subdivisions include the hypothalamus to be discussed in a separate lecture, the ventral thalamus containing the subthalamic nucleus already discussed, the epithalamus which is made up mostly of the pineal body, and the dorsal thalamus (henceforth referred to as the thalamus) which is the focus of this lecture. Although we will not spend any time in lecture on the pineal body, part of the epithalamus, it does have some interesting features as well as some clinical relevance. The pineal is a small midline mass of glandular tissue that secretes the hormone melatonin. In lower mammals, melatonin plays a central role in control of diurnal rhythms (cycles in body states and hormone levels that follow the day- night cycle). In humans, at least a portion of the control of diurnal rhythms has been taken over by the hypothalamus, but there is increasing evidence that the pineal and melatonin play at least a limited role. Recent investigations have demonstrated a role for melatonin in sleep, tumor reduction and aging. Additionally, based on the observation that tumors of the pineal can induce a precocious puberty in males it has been suggested that the pineal is also involved in timing the onset of puberty. In many individuals the pineal is partially calcified and can serve as a marker for the midline of the brain on x- rays. Pathological processes can sometimes be detected by a shift in its position. -

Brain Anatomy
BRAIN ANATOMY Adapted from Human Anatomy & Physiology by Marieb and Hoehn (9th ed.) The anatomy of the brain is often discussed in terms of either the embryonic scheme or the medical scheme. The embryonic scheme focuses on developmental pathways and names regions based on embryonic origins. The medical scheme focuses on the layout of the adult brain and names regions based on location and functionality. For this laboratory, we will consider the brain in terms of the medical scheme (Figure 1): Figure 1: General anatomy of the human brain Marieb & Hoehn (Human Anatomy and Physiology, 9th ed.) – Figure 12.2 CEREBRUM: Divided into two hemispheres, the cerebrum is the largest region of the human brain – the two hemispheres together account for ~ 85% of total brain mass. The cerebrum forms the superior part of the brain, covering and obscuring the diencephalon and brain stem similar to the way a mushroom cap covers the top of its stalk. Elevated ridges of tissue, called gyri (singular: gyrus), separated by shallow groves called sulci (singular: sulcus) mark nearly the entire surface of the cerebral hemispheres. Deeper groves, called fissures, separate large regions of the brain. Much of the cerebrum is involved in the processing of somatic sensory and motor information as well as all conscious thoughts and intellectual functions. The outer cortex of the cerebrum is composed of gray matter – billions of neuron cell bodies and unmyelinated axons arranged in six discrete layers. Although only 2 – 4 mm thick, this region accounts for ~ 40% of total brain mass. The inner region is composed of white matter – tracts of myelinated axons. -

Diencephalon and Hypothalamus
Diencephalon and Hypothalamus Objectives: 1) To become familiar with the four major divisions of the diencephalon 2) To understand the major anatomical divisions and functions of the hypothalamus. 3) To appreciate the relationship of the hypothalamus to the pituitary gland Four Subdivisions of the Diencephalon: Epithalamus, Subthalamus Thalamus & Hypothalamus Epithalamus 1. Epithalamus — (“epi” means upon) the most dorsal part of the diencephalon; it forms a caplike covering over the thalamus. a. The smallest and oldest part of the diencephalon b. Composed of: pineal body, habenular nuclei and the caudal commissure c. Function: It is functionally and anatomically linked to the limbic system; implicated in a number of autonomic (ie. respiratory, cardio- vascular), endocrine (thyroid function) and reproductive (mating behavior; responsible for postpartum maternal behavior) functions. Melatonin is secreted by the pineal gland at night and is concerned with biological timing including sleep induction. 2. Subthalamus — (“sub” = below), located ventral to the thalamus and lateral to the hypothalamus (only present in mammals). a. Plays a role in the generation of rhythmic movements b. Recent work indicates that stimulation of the subthalamus in cats inhibits the micturition reflex and thus this nucleus may also be involved in neural control of micturition. c. Stimulation of the subthalamus provides the most effective treatment for late-stage Parkinson’s disease in humans. Subthalamus 3. Thalamus — largest component of the diencephalon a. comprised of a large number of nuclei; -->lateral geniculate (vision) and the medial geniculate (hearing). b. serves as the great sensory receiving area (receives sensory input from all sensory pathways except olfaction) and relays sensory information to the cerebral cortex. -

The Thalamus Drives Light-Evoked Activity in the Habenula of Larval
bioRxiv preprint doi: https://doi.org/10.1101/047936; this version posted December 17, 2016. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 The thalamus drives light-evoked activity in the habenula of 2 larval zebrafish 3 Ruey-Kuang Cheng1, Seetha Krishnan2, Qian Lin2, David G. C. Hildebrand3, 4, Isaac H. 4 Bianco4 and *Suresh Jesuthasan1, 5, 6, 7 5 1Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore 6 636921. 7 2NUS Graduate School for Integrative Sciences and Engineering, 28 Medical Drive, 8 National University of Singapore, Singapore 117456. 9 3Graduate Program in Neuroscience, Division of Medical Sciences, Graduate School of 10 Arts and Sciences, Harvard University, Cambridge, Massachusetts 02138 United States 11 of America. 12 4Department of Molecular and Cell Biology, Harvard University, Cambridge, 13 Massachusetts 02138, United States of America. 14 5Neural Circuitry and Behavior Laboratory, Institute of Molecular and Cell Biology, 15 Singapore 138673. 16 6Neuroscience and Behavioral Disorders Program, Duke-NUS Graduate Medical School, 17 8 College Road, Singapore 169857. 18 7Department of Physiology, National University of Singapore, Singapore 117597. 19 *Corresponding author: [email protected]; +65 65869545 20 equal contribution 1 bioRxiv preprint doi: https://doi.org/10.1101/047936; this version posted December 17, 2016. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 21 Abstract 22 The habenula integrates sensory stimuli and reward information to regulate the 23 release of neuromodulators with broad effects on brain state and behavior. -

White Matter Anatomy: What the Radiologist Needs to Know
White Matter Anatomy What the Radiologist Needs to Know Victor Wycoco, MBBS, FRANZCRa, Manohar Shroff, MD, DABR, FRCPCa,*, Sniya Sudhakar, MBBS, DNB, MDb, Wayne Lee, MSca KEYWORDS Diffusion tensor imaging (DTI) White matter tracts Projection fibers Association Fibers Commissural fibers KEY POINTS Diffusion tensor imaging (DTI) has emerged as an excellent tool for in vivo demonstration of white matter microstructure and has revolutionized our understanding of the same. Information on normal connectivity and relations of different white matter networks and their role in different disease conditions is still evolving. Evidence is mounting on causal relations of abnormal white matter microstructure and connectivity in a wide range of pediatric neurocognitive and white matter diseases. Hence there is a pressing need for every neuroradiologist to acquire a strong basic knowledge of white matter anatomy and to make an effort to apply this knowledge in routine reporting. INTRODUCTION (Fig. 1). However, the use of specific DTI sequences provides far more detailed and clini- DTI has allowed in vivo demonstration of axonal cally useful information. architecture and connectivity. This technique has set the stage for numerous studies on normal and abnormal connectivity and their role in devel- DIFFUSION TENSOR IMAGING: THE BASICS opmental and acquired disorders. Referencing established white matter anatomy, DTI atlases, Using appropriate magnetic field gradients, and neuroanatomical descriptions, this article diffusion-weighted sequences can be used to summarizes the major white matter anatomy and detect the motion of the water molecules to and related structures relevant to the clinical neurora- from cells. This free movement of the water mole- diologist in daily practice. -

The Sensory Axis
The sensory axis By Gustav Emil Dietrichson Things I’m going to cover and you’re going to understand • The basics • Cutaneous receptors • Dorsal column-medial lemniscus • Spinothalamic tract • The thalamus and the cortex • Pain • Quesons Receptors • Smulus à Conducon change à Generator poten?al • Intereceptors, exteroceptors, proprioceptors, teleceptors Thermoreceptors Chemoreceptors Photoreceptors Mechanoreceptors Nerve fibers • A • B – Preganglionic • C – Pain, temp • Aα – Propriocep?on autonomic ggl • 0,5-2m/s • 70-120m/s • 3-12m/s • Cggl – Postsynap?c • Aβ – Touch sympathe?ch ggl • 5-12m/s • 0,7-2,3m/s • Aγ – Motor • 3-6m/s • Aδ – Pain, temp • 12-30m/s A fibers = Thickest C fibers = Thinnest Cutaneous receptors • Merkel discs • Ruffini endings • Fine touch • Skin stretch • Discriminave touch Apical • Sustained pressure Basal • Meissner corpuscles • Pacinian corpuscles • Texture change • Deep touch • Slow vibraons • Fast vibraons • Both ”Corpuscles” are PHASIC receptors, while the other two are TONIC receptors • *ALL USE Aβ FIBERS Thalamus • What is the Thalamus? • Ventroposterolateral nucleusàBrodmann area 3,1,2 Dorsal column- medial lemniscus • Receives all informaon from • Merkel discs, Meissner corpuscles, Pacinian corpuscles, Ruffini endings • Muscles spindles and Golgi tendon organs • Propriocep?on • Fine touch • Vibraon (Low and high) • Pressure (deep and superficial) • Two touch discriminaon • DRAW! Spinothalamic tract Lateral spinothalamic tract • Anterior spinothalamic • Crude touch • Lateral spinothalamic • Temperature • Pain Everything