Habenula and Thalamus Cell Transplants Mediate Different Specific Patterns of Innervation in the Lnterpeduncular Nucleus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cortex and Thalamus Lecture.Pptx
Cerebral Cortex and Thalamus Hyperbrain Ch 2 Monica Vetter, PhD January 24, 2013 Learning Objectives: • Anatomy of the lobes of the cortex • Relationship of thalamus to cortex • Layers and connectivity of the cortex • Vascular supply to cortex • Understand the location and function of hypothalamus and pituitary • Anatomy of the basal ganglia • Primary functions of the different lobes/ cortical regions – neurological findings 1 Types of Cortex • Sensory (Primary) • Motor (Primary) • Unimodal association • Multimodal association - necessary for language, reason, plan, imagine, create Note: • Gyri • Sulci • Fissures • Lobes 2 The Thalamus is highly interconnected with the cerebral cortex, and handles most information traveling to or from the cortex. “Specific thalamic Ignore nuclei” – have well- names of defined sensory or thalamic nuclei for motor functions now - A few Other nuclei have will more distributed reappear later function 3 Thalamus Midbrain Pons Limbic lobe = cingulate gyrus Structure of Neocortex (6 layers) white matter gray matter Pyramidal cells 4 Connectivity of neurons in different cortical layers Afferents = inputs Efferents = outputs (reciprocal) brainstem etc Eg. Motor – Eg. Sensory – more efferent more afferent output input Cortico- cortical From Thalamus To spinal cord, brainstem etc. To Thalamus Afferent and efferent connections to different ….Depending on whether they have more layers of cortex afferent or efferent connections 5 Different areas of cortex were defined by differences in layer thickness, and size and -

The Human Thalamus Is an Integrative Hub for Functional Brain Networks
5594 • The Journal of Neuroscience, June 7, 2017 • 37(23):5594–5607 Behavioral/Cognitive The Human Thalamus Is an Integrative Hub for Functional Brain Networks X Kai Hwang, Maxwell A. Bertolero, XWilliam B. Liu, and XMark D’Esposito Helen Wills Neuroscience Institute and Department of Psychology, University of California, Berkeley, Berkeley, California 94720 The thalamus is globally connected with distributed cortical regions, yet the functional significance of this extensive thalamocortical connectivityremainslargelyunknown.Byperforminggraph-theoreticanalysesonthalamocorticalfunctionalconnectivitydatacollected from human participants, we found that most thalamic subdivisions display network properties that are capable of integrating multi- modal information across diverse cortical functional networks. From a meta-analysis of a large dataset of functional brain-imaging experiments, we further found that the thalamus is involved in multiple cognitive functions. Finally, we found that focal thalamic lesions in humans have widespread distal effects, disrupting the modular organization of cortical functional networks. This converging evidence suggests that the human thalamus is a critical hub region that could integrate diverse information being processed throughout the cerebral cortex as well as maintain the modular structure of cortical functional networks. Key words: brain networks; diaschisis; functional connectivity; graph theory; thalamus Significance Statement The thalamus is traditionally viewed as a passive relay station of information from sensory organs or subcortical structures to the cortex. However, the thalamus has extensive connections with the entire cerebral cortex, which can also serve to integrate infor- mation processing between cortical regions. In this study, we demonstrate that multiple thalamic subdivisions display network properties that are capable of integrating information across multiple functional brain networks. Moreover, the thalamus is engaged by tasks requiring multiple cognitive functions. -

Basal Ganglia Anatomy, Physiology, and Function Ns201c
Basal Ganglia Anatomy, Physiology, and Function NS201c Human Basal Ganglia Anatomy Basal Ganglia Circuits: The ‘Classical’ Model of Direct and Indirect Pathway Function Motor Cortex Premotor Cortex + Glutamate Striatum GPe GPi/SNr Dopamine + - GABA - Motor Thalamus SNc STN Analagous rodent basal ganglia nuclei Gross anatomy of the striatum: gateway to the basal ganglia rodent Dorsomedial striatum: -Inputs predominantly from mPFC, thalamus, VTA Dorsolateral striatum: -Inputs from sensorimotor cortex, thalamus, SNc Ventral striatum: Striatal subregions: Dorsomedial (caudate) -Inputs from vPFC, hippocampus, amygdala, Dorsolateral (putamen) thalamus, VTA Ventral (nucleus accumbens) Gross anatomy of the striatum: patch and matrix compartments Patch/Striosome: -substance P -mu-opioid receptor Matrix: -ChAT and AChE -somatostatin Microanatomy of the striatum: cell types Projection neurons: MSN: medium spiny neuron (GABA) •striatonigral projecting – ‘direct pathway’ •striatopallidal projecting – ‘indirect pathway’ Interneurons: FS: fast-spiking interneuron (GABA) LTS: low-threshold spiking interneuron (GABA) LA: large aspiny neuron (ACh) 30 um Cellular properties of striatal neurons Microanatomy of the striatum: striatal microcircuits • Feedforward inhibition (mediated by fast-spiking interneurons) • Lateral feedback inhibition (mediated by MSN collaterals) Basal Ganglia Circuits: The ‘Classical’ Model of Direct and Indirect Pathway Function Motor Cortex Premotor Cortex + Glutamate Striatum GPe GPi/SNr Dopamine + - GABA - Motor Thalamus SNc STN The simplified ‘classical’ model of basal ganglia circuit function • Information encoded as firing rate • Basal ganglia circuit is linear and unidirectional • Dopamine exerts opposing effects on direct and indirect pathway MSNs Basal ganglia motor circuit: direct pathway Motor Cortex Premotor Cortex Glutamate Striatum GPe GPi/SNr Dopamine + GABA Motor Thalamus SNc STN Direct pathway MSNs express: D1, M4 receptors, Sub. -

Clones in the Chick Diencephalon Contain Multiple Cell Types and Siblings Are Widely Dispersed
Development 122, 65-78 (1996) 65 Printed in Great Britain © The Company of Biologists Limited 1996 DEV8292 Clones in the chick diencephalon contain multiple cell types and siblings are widely dispersed Jeffrey A. Golden1,2 and Constance L. Cepko1,3 1Department of Genetics, Harvard Medical School, 2Department of Pathology, Brigham and Women’s Hospital, and 3Howard Hughes Medical Institute, 200 Longwood Avenue, Boston, MA 02115, USA SUMMARY The thalamus, hypothalamus and epithalamus of the ver- clones dispersed in all directions, resulting in sibling cells tebrate central nervous system are derived from the populating multiple nuclei within the diencephalon. In embryonic diencephalon. These regions of the nervous addition, several distinctive patterns of dispersion were system function as major relays between the telencephalon observed. These included clones with siblings distributed and more caudal regions of the brain. Early in develop- bilaterally across the third ventricle, clones that originated ment, the diencephalon morphologically comprises distinct in the lateral ventricle, clones that crossed neuromeric units known as neuromeres or prosomeres. As development boundaries, and clones that crossed major boundaries of proceeds, multiple nuclei, the functional and anatomical the developing nervous system, such as the diencephalon units of the diencephalon, derive from the neuromeres. It and mesencephalon. These findings demonstrate that prog- was of interest to determine whether progenitors in the enitor cells in the diencephalon are multipotent and that diencephalon give rise to daughters that cross nuclear or their daughters can become widely dispersed. neuromeric boundaries. To this end, a highly complex retroviral library was used to infect diencephalic progeni- tors. Retrovirally marked clones were found to contain Key words: cell lineage, central nervous system, diencephalon, neurons, glia and occasionally radial glia. -

The Thalamus: Gateway to the Mind Lawrence M
Overview The thalamus: gateway to the mind Lawrence M. Ward∗ The thalamus of the brain is far more than the simple sensory relay it was long thought to be. From its location at the top of the brain stem it interacts directly with nearly every part of the brain. Its dense loops into and out of cortex render it functionally a seventh cortical layer. Moreover, it receives and sends connections to most subcortical areas as well. Of course it does function as a very sophisticated sensory relay and thus is of vital importance to perception. But also it functions critically in all mental operations, including attention, memory, and consciousness, likely in different ways for different processes, as indicated by the consequences of damage to its various nuclei as well as by invasive studies in nonhuman animals. It plays a critical role also in the arousal system of the brain, in emotion, in movement, and in coordinating cortical computations. Given these important functional roles, and the dearth of knowledge about the details of its nonsensory nuclei, it is an attractive target for intensive study in the future, particularly in regard to its role in healthy and impaired cognitive functioning. © 2013 John Wiley & Sons, Ltd. How to cite this article: WIREs Cogn Sci 2013. doi: 10.1002/wcs.1256 INTRODUCTION and other animals can do quite well without major chunks of cortex. Indeed, decorticate rats behave very pen nearly any textbook of neuroscience or similarly to normal rats in many ways,2 whereas sensation and perception and you will find the O de-thalamate rats die. -

Lmmunohistochemical Localization of Neuronal Nicotinic Receptors in the Rodent Central Nervous System
The Journal of Neuroscience, October 1987, 7(10): 3334-3342 lmmunohistochemical Localization of Neuronal Nicotinic Receptors in the Rodent Central Nervous System L. W. Swanson,1v2 D. M. Simmons, I12 P. J. Whiting,’ and J. Lindstrom’ ‘The Salk Institute for Biological Studies, and 2Howard Hughes Medical Institute, La Jolla, California 92037 The distribution of nicotinic acetylcholine receptors (AChR) are structurally unrelated (Kubo et al., 1986a, b) to nicotinic in the rat and mouse central nervous system has been ACh receptors (AChR, Noda et al., 1983a, b), which act by mapped in detail using monoclonal antibodies to receptors regulating directly the opening of a cation channel that is an purified from chicken and rat brain. Initial studies in the intrinsic component of the molecule. Furthermore, subtypes of chicken brain indicate that different neuronal AChRs are con- neuronal AChRs have been identified on the basis of pharma- tained in axonal projections to the optic lobe in the midbrain cological and structural properties (Whiting et al., 1987a). To from neurons in the lateral spiriform nucleus and from retinal understand the functional significanceof ACh in a particular ganglion cells. Monoclonal antibodies to the chicken and rat neural system, it is therefore necessaryto establishthe cellular brain AChRs also label apparently identical regions in all localization of ACh, and the cellular localization and type of major subdivisions of the central nervous system of rats and cholinergic receptor with which it interacts. Immunohistochem- mice, and this pattern is very similar to previous reports of istry provides a sensitive method for localizing cholinergic neu- 3H-nicotine binding, but quite different from that of a-bun- rons with antibodies to the synthetic enzyme choline acetyl- garotoxin binding. -

Tonic Activity in Lateral Habenula Neurons Promotes Disengagement from Reward-Seeking Behavior
bioRxiv preprint doi: https://doi.org/10.1101/2021.01.15.426914; this version posted January 16, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Tonic activity in lateral habenula neurons promotes disengagement from reward-seeking behavior 5 Brianna J. Sleezer1,3, Ryan J. Post1,2,3, David A. Bulkin1,2,3, R. Becket Ebitz4, Vladlena Lee1, Kasey Han1, Melissa R. Warden1,2,5,* 1Department of Neurobiology and Behavior, Cornell University, Ithaca, NY 14853, USA 2Cornell Neurotech, Cornell University, Ithaca, NY 14853 USA 10 3These authors contributed equally 4Department oF Neuroscience, Université de Montréal, Montréal, QC H3T 1J4, Canada 5Lead Contact *Correspondence: [email protected] 15 Sleezer*, Post*, Bulkin* et al. 1 of 38 bioRxiv preprint doi: https://doi.org/10.1101/2021.01.15.426914; this version posted January 16, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. SUMMARY Survival requires both the ability to persistently pursue goals and the ability to determine when it is time to stop, an adaptive balance of perseverance and disengagement. Neural activity in the 5 lateral habenula (LHb) has been linked to aversion and negative valence, but its role in regulating the balance between reward-seeking and disengaged behavioral states remains unclear. -

Retrograde Inhibition by a Specific Subset of Interpeduncular Α5 Nicotinic Neurons Regulates Nicotine Preference
Retrograde inhibition by a specific subset of interpeduncular α5 nicotinic neurons regulates nicotine preference Jessica L. Ablesa,b,c, Andreas Görlicha,1, Beatriz Antolin-Fontesa,2,CuidongWanga, Sylvia M. Lipforda, Michael H. Riada, Jing Rend,e,3,FeiHud,e,4,MinminLuod,e,PaulJ.Kennyc, Nathaniel Heintza,f,5, and Ines Ibañez-Tallona,5 aLaboratory of Molecular Biology, The Rockefeller University, New York, NY 10065; bDepartment of Psychiatry, Icahn School of Medicine at Mount Sinai, New York, NY 10029; cDepartment of Neuroscience, Icahn School of Medicine at Mount Sinai, New York, NY 10029; dNational Institute of Biological Sciences, Beijing 102206, China; eSchool of Life Sciences, Tsinghua University, Beijing 100084, China; and fHoward Hughes Medical Institute, The Rockefeller University, New York, NY 10065 Contributed by Nathaniel Heintz, October 23, 2017 (sent for review October 5, 2017; reviewed by Jean-Pierre Changeux and Lorna W. Role) Repeated exposure to drugs of abuse can produce adaptive changes nicotine withdrawal, and optical activation of IPN GABAergic cells that lead to the establishment of dependence. It has been shown that is sufficient to produce a withdrawal syndrome, while blockade of allelic variation in the α5 nicotinic acetylcholine receptor (nAChR) gene GABAergic cells in the IPN reduced symptoms of withdrawal (17). CHRNA5 is associated with higher risk of tobacco dependence. In the Taken together these studies highlight the critical role of α5in brain, α5-containing nAChRs are expressed at very high levels in the regulating behavioral responses to nicotine. Here we characterize two subpopulations of GABAergic interpeduncular nucleus (IPN). Here we identified two nonoverlapping Amigo1 Epyc α + α Amigo1 α Epyc neurons in the IPN that express α5: α5- and α5- neu- 5 cell populations ( 5- and 5- ) in mouse IPN that respond α Amigo1 α Epyc differentially to nicotine. -
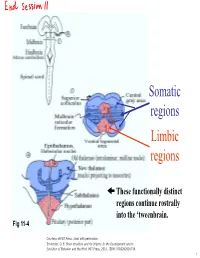
Lecture 12 Notes
Somatic regions Limbic regions These functionally distinct regions continue rostrally into the ‘tweenbrain. Fig 11-4 Courtesy of MIT Press. Used with permission. Schneider, G. E. Brain structure and its Origins: In the Development and in Evolution of Behavior and the Mind. MIT Press, 2014. ISBN: 9780262026734. 1 Chapter 11, questions about the somatic regions: 4) There are motor neurons located in the midbrain. What movements do those motor neurons control? (These direct outputs of the midbrain are not a subject of much discussion in the chapter.) 5) At the base of the midbrain (ventral side) one finds a fiber bundle that shows great differences in relative size in different species. Give examples. What are the fibers called and where do they originate? 8) A decussating group of axons called the brachium conjunctivum also varies greatly in size in different species. It is largest in species with the largest neocortex but does not come from the neocortex. From which structure does it come? Where does it terminate? (Try to guess before you look it up.) 2 Motor neurons of the midbrain that control somatic muscles: the oculomotor nuclei of cranial nerves III and IV. At this level, the oculomotor nucleus of nerve III is present. Fibers from retina to Superior Colliculus Brachium of Inferior Colliculus (auditory pathway to thalamus, also to SC) Oculomotor nucleus Spinothalamic tract (somatosensory; some fibers terminate in SC) Medial lemniscus Cerebral peduncle: contains Red corticospinal + corticopontine fibers, + cortex to hindbrain fibers nucleus (n. ruber) Tectospinal tract Rubrospinal tract Courtesy of MIT Press. Used with permission. Schneider, G. -

Brain Structure and Function Related to Headache
Review Cephalalgia 0(0) 1–26 ! International Headache Society 2018 Brain structure and function related Reprints and permissions: sagepub.co.uk/journalsPermissions.nav to headache: Brainstem structure and DOI: 10.1177/0333102418784698 function in headache journals.sagepub.com/home/cep Marta Vila-Pueyo1 , Jan Hoffmann2 , Marcela Romero-Reyes3 and Simon Akerman3 Abstract Objective: To review and discuss the literature relevant to the role of brainstem structure and function in headache. Background: Primary headache disorders, such as migraine and cluster headache, are considered disorders of the brain. As well as head-related pain, these headache disorders are also associated with other neurological symptoms, such as those related to sensory, homeostatic, autonomic, cognitive and affective processing that can all occur before, during or even after headache has ceased. Many imaging studies demonstrate activation in brainstem areas that appear specifically associated with headache disorders, especially migraine, which may be related to the mechanisms of many of these symptoms. This is further supported by preclinical studies, which demonstrate that modulation of specific brainstem nuclei alters sensory processing relevant to these symptoms, including headache, cranial autonomic responses and homeostatic mechanisms. Review focus: This review will specifically focus on the role of brainstem structures relevant to primary headaches, including medullary, pontine, and midbrain, and describe their functional role and how they relate to mechanisms -

Nicotine Aversion Is Mediated by Gabaergic Interpeduncular Nucleus Inputs to Laterodorsal Tegmentum
ARTICLE DOI: 10.1038/s41467-018-04654-2 OPEN Nicotine aversion is mediated by GABAergic interpeduncular nucleus inputs to laterodorsal tegmentum Shannon L. Wolfman1, Daniel F. Gill1, Fili Bogdanic2, Katie Long3, Ream Al-Hasani4, Jordan G. McCall4,5,6, Michael R. Bruchas5,6 & Daniel S. McGehee1,2 1234567890():,; Nicotine use can lead to dependence through complex processes that are regulated by both its rewarding and aversive effects. Recent studies show that aversive nicotine doses activate excitatory inputs to the interpeduncular nucleus (IPN) from the medial habenula (MHb), but the downstream targets of the IPN that mediate aversion are unknown. Here we show that IPN projections to the laterodorsal tegmentum (LDTg) are GABAergic using optoge- netics in tissue slices from mouse brain. Selective stimulation of these IPN axon terminals in LDTg in vivo elicits avoidance behavior, suggesting that these projections contribute to aversion. Nicotine modulates these synapses in a concentration-dependent manner, with strong enhancement only seen at higher concentrations that elicit aversive responses in behavioral tests. Optogenetic inhibition of the IPN–LDTg connection blocks nicotine condi- tioned place aversion, suggesting that the IPN–LDTg connection is a critical part of the circuitry that mediates the aversive effects of nicotine. 1 Committee on Neurobiology, University of Chicago, Chicago, IL 60637, USA. 2 Department of Anesthesia & Critical Care, University of Chicago, Chicago, IL 60637, USA. 3 Interdisciplinary Scientist Training Program, University of Chicago, Chicago, IL 60637, USA. 4 St. Louis College of Pharmacy, Center for Clinical Pharmacology and Division of Basic Research of the Department of Anesthesiology, Washington University School of Medicine, St. -

Loss of the Habenula Intrinsic Neuromodulator Kisspeptin1 Affects Learning in Larval Zebrafish
This document is downloaded from DR‑NTU (https://dr.ntu.edu.sg) Nanyang Technological University, Singapore. Loss of the habenula intrinsic neuromodulator Kisspeptin1 affects learning in larval zebrafish Lupton, Charlotte; Sengupta, Mohini; Cheng, Ruey‑Kuang; Chia, Joanne; Thirumalai, Vatsala; Jesuthasan, Suresh 2017 Lupton, C., Sengupta, M., Cheng, R.‑K., Chia, J., Thirumalai, V., & Jesuthasan, S. (2017). Loss of the habenula intrinsic neuromodulator Kisspeptin1 affects learning in larval zebrafish. eNeuro, 4(3), e0326‑16.2017‑. doi:10.1523/ENEURO.0326‑16.2017 https://hdl.handle.net/10356/107013 https://doi.org/10.1523/ENEURO.0326‑16.2017 © 2017 Lupton et al. This is an open‑access article distributed under the terms of the Creative Commons Attribution 4.0 International license, which permits unrestricted use, distribution and reproduction in any medium provided that the original work is properly attributed. Downloaded on 29 Sep 2021 15:54:33 SGT New Research Cognition and Behavior Loss of the Habenula Intrinsic Neuromodulator Kisspeptin1 Affects Learning in Larval Zebrafish Joanne Chia,5 Vatsala ء,Ruey-Kuang Cheng,4 ء,Mohini Sengupta,3 ء,Charlotte Lupton,1,2 and Suresh Jesuthasan2,4,6 ء,Thirumalai,3 DOI:http://dx.doi.org/10.1523/ENEURO.0326-16.2017 1Department of Animal and Plant Sciences, University of Sheffield, Sheffield, S10 2TN, UK, 2Institute for Molecular and Cell Biology, 138673, Singapore, 3National Centre for Biological Sciences, Tata Institute of Fundamental Research, Bangalore, 560065, India, 4Lee Kong Chian School of Medicine, Nanyang Technological University, 636921, Singapore, 5National University of Singapore Graduate School for Integrative Sciences and Engineering, 117456, Singapore, and 6Duke-NUS Graduate Medical School, 169857 Singapore Abstract Learning how to actively avoid a predictable threat involves two steps: recognizing the cue that predicts upcoming punishment and learning a behavioral response that will lead to avoidance.