Note on Crumb's "Liberae Et Confluentae" Couplet (Noctuidae)1.2
Total Page:16
File Type:pdf, Size:1020Kb
Recommended publications
-

Lepidoptera of North America 5
Lepidoptera of North America 5. Contributions to the Knowledge of Southern West Virginia Lepidoptera Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Lepidoptera of North America 5. Contributions to the Knowledge of Southern West Virginia Lepidoptera by Valerio Albu, 1411 E. Sweetbriar Drive Fresno, CA 93720 and Eric Metzler, 1241 Kildale Square North Columbus, OH 43229 April 30, 2004 Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Cover illustration: Blueberry Sphinx (Paonias astylus (Drury)], an eastern endemic. Photo by Valeriu Albu. ISBN 1084-8819 This publication and others in the series may be ordered from the C.P. Gillette Museum of Arthropod Diversity, Department of Bioagricultural Sciences and Pest Management Colorado State University, Fort Collins, CO 80523 Abstract A list of 1531 species ofLepidoptera is presented, collected over 15 years (1988 to 2002), in eleven southern West Virginia counties. A variety of collecting methods was used, including netting, light attracting, light trapping and pheromone trapping. The specimens were identified by the currently available pictorial sources and determination keys. Many were also sent to specialists for confirmation or identification. The majority of the data was from Kanawha County, reflecting the area of more intensive sampling effort by the senior author. This imbalance of data between Kanawha County and other counties should even out with further sampling of the area. Key Words: Appalachian Mountains, -

Moths of Ohio Guide
MOTHS OF OHIO field guide DIVISION OF WILDLIFE This booklet is produced by the ODNR Division of Wildlife as a free publication. This booklet is not for resale. Any unauthorized INTRODUCTION reproduction is prohibited. All images within this booklet are copyrighted by the Division of Wildlife and it’s contributing artists and photographers. For additional information, please call 1-800-WILDLIFE. Text by: David J. Horn Ph.D Moths are one of the most diverse and plentiful HOW TO USE THIS GUIDE groups of insects in Ohio, and the world. An es- Scientific Name timated 160,000 species have thus far been cata- Common Name Group and Family Description: Featured Species logued worldwide, and about 13,000 species have Secondary images 1 Primary Image been found in North America north of Mexico. Secondary images 2 Occurrence We do not yet have a clear picture of the total Size: when at rest number of moth species in Ohio, as new species Visual Index Ohio Distribution are still added annually, but the number of species Current Page Description: Habitat & Host Plant is certainly over 3,000. Although not as popular Credit & Copyright as butterflies, moths are far more numerous than their better known kin. There is at least twenty Compared to many groups of animals, our knowledge of moth distribution is very times the number of species of moths in Ohio as incomplete. Many areas of the state have not been thoroughly surveyed and in some there are butterflies. counties hardly any species have been documented. Accordingly, the distribution maps in this booklet have three levels of shading: 1. -

Series I. Correspondence, 1871-1894 Box 1 Folder 1 Darwin to Riley
Special Collections at the National Agricultural Library: Charles Valentine Riley Collection Series I. Correspondence, 1871-1894 Box 1 Folder 1 Darwin to Riley. June 1, 1871. Letter from Charles Darwin to Riley thanking him for report and instructions on noxious insects. Downs, Beckerham, Kent (England). (handwritten copy of original). Box 1 Folder 2 Koble to Riley. June 30, 1874. Letter from John C. Koble giving physical description of chinch bugs and explaining how the bugs are destroying corn crops in western Kentucky. John C. Koble of L. S. Trimble and Co., Bankers. Box 1 Folder 3 Saunders to Riley. Nov. 12, 1874. William Saunders receipt to C. V. Riley for a copy of descriptions of two insects that baffle the vegetable carnivora. William Saunders, Department of Agriculture, Washington, D. C. Box 1 Folder 4 Young to Riley. Dec. 13, 1874. William Young describes the flat-headed borer and its effects on orchards during summer and winter seasons. From Palmyra Gate Co., Nebraska. Box 1 Folder 5 Saunders to Riley. Dec. 22, 1874. William Saunders receipt of notes of investigation on the insects associated with Sarracenia. William Saunders, Department of Agriculture, Washington, D.C. Box 1 Folder 6 Bonhaw to Riley. Jan. 19, 1875. L. N. Bonhaw requesting a copy of his Missouri report, for him to establish a manual or handbook on entomology, and to find out about an insect that deposits eggs. Subject: tomato worm, hawk moth. 1 http://www.nal.usda.gov/speccoll/ Special Collections at the National Agricultural Library: Charles Valentine Riley Collection Box 1 Folder 7 Holliday to Riley. -

1 Modern Threats to the Lepidoptera Fauna in The
MODERN THREATS TO THE LEPIDOPTERA FAUNA IN THE FLORIDA ECOSYSTEM By THOMSON PARIS A THESIS PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE UNIVERSITY OF FLORIDA 2011 1 2011 Thomson Paris 2 To my mother and father who helped foster my love for butterflies 3 ACKNOWLEDGMENTS First, I thank my family who have provided advice, support, and encouragement throughout this project. I especially thank my sister and brother for helping to feed and label larvae throughout the summer. Second, I thank Hillary Burgess and Fairchild Tropical Gardens, Dr. Jonathan Crane and the University of Florida Tropical Research and Education center Homestead, FL, Elizabeth Golden and Bill Baggs Cape Florida State Park, Leroy Rogers and South Florida Water Management, Marshall and Keith at Mack’s Fish Camp, Susan Casey and Casey’s Corner Nursery, and Michael and EWM Realtors Inc. for giving me access to collect larvae on their land and for their advice and assistance. Third, I thank Ryan Fessendon and Lary Reeves for helping to locate sites to collect larvae and for assisting me to collect larvae. I thank Dr. Marc Minno, Dr. Roxanne Connely, Dr. Charles Covell, Dr. Jaret Daniels for sharing their knowledge, advice, and ideas concerning this project. Fourth, I thank my committee, which included Drs. Thomas Emmel and James Nation, who provided guidance and encouragement throughout my project. Finally, I am grateful to the Chair of my committee and my major advisor, Dr. Andrei Sourakov, for his invaluable counsel, and for serving as a model of excellence of what it means to be a scientist. -

Illustration Sources
APPENDIX ONE ILLUSTRATION SOURCES REF. CODE ABR Abrams, L. 1923–1960. Illustrated flora of the Pacific states. Stanford University Press, Stanford, CA. ADD Addisonia. 1916–1964. New York Botanical Garden, New York. Reprinted with permission from Addisonia, vol. 18, plate 579, Copyright © 1933, The New York Botanical Garden. ANDAnderson, E. and Woodson, R.E. 1935. The species of Tradescantia indigenous to the United States. Arnold Arboretum of Harvard University, Cambridge, MA. Reprinted with permission of the Arnold Arboretum of Harvard University. ANN Hollingworth A. 2005. Original illustrations. Published herein by the Botanical Research Institute of Texas, Fort Worth. Artist: Anne Hollingworth. ANO Anonymous. 1821. Medical botany. E. Cox and Sons, London. ARM Annual Rep. Missouri Bot. Gard. 1889–1912. Missouri Botanical Garden, St. Louis. BA1 Bailey, L.H. 1914–1917. The standard cyclopedia of horticulture. The Macmillan Company, New York. BA2 Bailey, L.H. and Bailey, E.Z. 1976. Hortus third: A concise dictionary of plants cultivated in the United States and Canada. Revised and expanded by the staff of the Liberty Hyde Bailey Hortorium. Cornell University. Macmillan Publishing Company, New York. Reprinted with permission from William Crepet and the L.H. Bailey Hortorium. Cornell University. BA3 Bailey, L.H. 1900–1902. Cyclopedia of American horticulture. Macmillan Publishing Company, New York. BB2 Britton, N.L. and Brown, A. 1913. An illustrated flora of the northern United States, Canada and the British posses- sions. Charles Scribner’s Sons, New York. BEA Beal, E.O. and Thieret, J.W. 1986. Aquatic and wetland plants of Kentucky. Kentucky Nature Preserves Commission, Frankfort. Reprinted with permission of Kentucky State Nature Preserves Commission. -

1 the RESTRUCTURING of ARTHROPOD TROPHIC RELATIONSHIPS in RESPONSE to PLANT INVASION by Adam B. Mitchell a Dissertation Submitt
THE RESTRUCTURING OF ARTHROPOD TROPHIC RELATIONSHIPS IN RESPONSE TO PLANT INVASION by Adam B. Mitchell 1 A dissertation submitted to the Faculty of the University of Delaware in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Entomology and Wildlife Ecology Winter 2019 © Adam B. Mitchell All Rights Reserved THE RESTRUCTURING OF ARTHROPOD TROPHIC RELATIONSHIPS IN RESPONSE TO PLANT INVASION by Adam B. Mitchell Approved: ______________________________________________________ Jacob L. Bowman, Ph.D. Chair of the Department of Entomology and Wildlife Ecology Approved: ______________________________________________________ Mark W. Rieger, Ph.D. Dean of the College of Agriculture and Natural Resources Approved: ______________________________________________________ Douglas J. Doren, Ph.D. Interim Vice Provost for Graduate and Professional Education I certify that I have read this dissertation and that in my opinion it meets the academic and professional standard required by the University as a dissertation for the degree of Doctor of Philosophy. Signed: ______________________________________________________ Douglas W. Tallamy, Ph.D. Professor in charge of dissertation I certify that I have read this dissertation and that in my opinion it meets the academic and professional standard required by the University as a dissertation for the degree of Doctor of Philosophy. Signed: ______________________________________________________ Charles R. Bartlett, Ph.D. Member of dissertation committee I certify that I have read this dissertation and that in my opinion it meets the academic and professional standard required by the University as a dissertation for the degree of Doctor of Philosophy. Signed: ______________________________________________________ Jeffery J. Buler, Ph.D. Member of dissertation committee I certify that I have read this dissertation and that in my opinion it meets the academic and professional standard required by the University as a dissertation for the degree of Doctor of Philosophy. -

Lepidoptera: Noctuidae) Species Plus Feeding Observations of Some Moths Common to Iowa William Hurston Hendrix III Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1990 Migration and behavioral studies of two adult noctuid (Lepidoptera: Noctuidae) species plus feeding observations of some moths common to Iowa William Hurston Hendrix III Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Botany Commons, Ecology and Evolutionary Biology Commons, and the Entomology Commons Recommended Citation Hendrix, William Hurston III, "Migration and behavioral studies of two adult noctuid (Lepidoptera: Noctuidae) species plus feeding observations of some moths common to Iowa " (1990). Retrospective Theses and Dissertations. 9373. https://lib.dr.iastate.edu/rtd/9373 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS The most advanced technology has been used to photograph and reproduce this manuscript from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. -

Common Butterflies and Moths (Order Lepidoptera) in the Wichita Mountains and Surrounding Areas
Common Butterflies and Moths (Order Lepidoptera) in the Wichita Mountains and Surrounding Areas Angel Chiri Less than 2% of known species in the U.S. have Entomologist approved common names. Relying on only common names for individual species may lead Introduction to confusion, since more than one common name may exist for the same species, or the With over 11,000 species described in the U.S. same name may be used for more than one and Canada, butterflies and moths are among the species. Using the scientific name, which is the most common and familiar insects. With few same in any language or region, eliminates this exceptions, the adults have two pairs of wings problem. Furthermore, only scientific names are covered with minute and easily dislodgeable used in the scientific literature. Common names scales. The mouthparts consist of a long, are not capitalized. flexible, and coiled proboscis that is used to absorb nectar. Butterflies and skippers are All photos in this guide were taken by the author diurnal, while most moths are nocturnal. using a Canon PowerShot SX110 IS camera. The Lepidoptera undergo a full metamorphosis. Family Pieridae (sulfurs and whites) The larva has a well developed head, with opposable mandibles designed for chewing and Pierids are common, mostly medium-sized, six simple eyes arranged in a semicircle, on each yellowish or white butterflies. The cloudless side of the head. The first three segments (the sulphur, Phoebis sennae has greenish-yellow or thorax) each bears a pair of segmented legs that lemon yellow wings with a spot resembling a end in a single claw. -
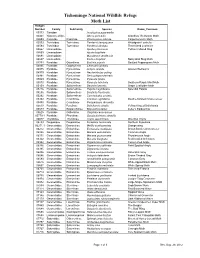
Tishomingo NWR Moth List
Tishomingo National Wildlife Refuge Moth List Hodges Number Family SubFamily Species Name_Common 00373 Tineidae Acrolophus popeanella 02401 Yponomeutidae Atteva punctella Ailanthus Webworm Moth 02693 Cossidae Cossinae Prionoxystus robinae Carpenterworm Moth 03593 Tortricidae Tortricinae Pandemis lamprosana Woodgrain Leafroller 03594 Tortricidae Tortricinae Pandemis limitata Three-lined Leafroller 04667 Limacodidae Apoda y-inversum Yellow-Collared Slug 04669 Limacodidae Apoda biguttata 04691 Limacodidae Monoleuca semifascia 04697 Limacodidae Euclea delphinii Spiny Oak Slug Moth 04794 Pyralidae Odontiinae Eustixia pupula Spotted Peppergrass Moth 04895 Pyralidae Glaphyriinae Chalcoela iphitalis 04975 Pyralidae Pyraustinae Achyra rantalis Garden Webworm 04979 Pyralidae Pyraustinae Neohelvibotys polingi 04991 Pyralidae Pyraustinae Sericoplaga externalis 05069 Pyralidae Pyraustinae Pyrausta tyralis 05070 Pyralidae Pyraustinae Pyrausta laticlavia Southern Purple Mint Moth 05159 Pyralidae Spilomelinae Desmia funeralis Grape Leaffolder Moth 05226 Pyralidae Spilomelinae Palpita magniferalis Splendid Palpita 05256 Pyralidae Spilomelinae Diastictis fracturalis 05292 Pyralidae Spilomelinae Conchylodes ovulalis 05362 Pyralidae Crambinae Crambus agitatellus Double-banded Grass-veneer 05450 Pyralidae Crambinae Parapediasia decorella 05533 Pyralidae Pyralinae Dolichomia olinalis Yellow-fringed Dolichomia 05579 Pyralidae Epipaschiinae Epipaschia zelleri Zeller's Epipaschia 05625 Pyralidae Galleriinae Omphalocera cariosa 05779.1 Pyralidae Phycitinae Quasisalebriaria -

Notes on the Life History of Two Sarbanissa Species (F Epidoptera: Noctuidae, Agaristinae) on the Malayan Peninsula 213- 228 ©Entomologischer Verein Apollo E.V
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Nachrichten des Entomologischen Vereins Apollo Jahr/Year: 1996 Band/Volume: 16 Autor(en)/Author(s): Rabenstein Renate, Speidel Wolfgang Artikel/Article: Notes on the life history of two Sarbanissa species (f epidoptera: Noctuidae, Agaristinae) on the Malayan peninsula 213- 228 ©Entomologischer Verein Apollo e.V. Frankfurt am Main; download unter www.zobodat.at Nachr. entomol. Ver. Apollo, N.F. 16 (2/3): 213-228 (1995) 213 Notes on the life history of two Sarbanissa species (f epidoptera: Noctuidae, Agaristinae) on the Malayan peninsula Rsnate R a b e n s t e in 1 and Wolfgang S p e id e l Dipl.-Biol. Renate Rabenstein, Abteilung Messelforschung, Forschungsinstitut Senckenberg, Serxkenberganlage 25, D-60325 Frankfurt/Main, Germany Dr Wolfgang Speidel, Zoologisches Fcrschungsinstitut und Museum Alexander Koenig, Adenauerallee 160, D-53113 Bonn, Germany Abstract: We report on the life history of Sarbanissa transiens (W a l k e r , 1856). The results of a change of the larval host-plant ( Cayratia mollissima, Vitaceae) to five other plant species of the same family are presented and the resulting effects on mortality, growth and development of the larvae are dis cussed. Furthermore we describe the larvae of S. catacoloides (W a l k e r , 1862) and give some information on their parasitoids (Hymenoptera: Braconidae). Our present knowledge on the host-plant specifity of Agaristinae larvae is reviewed. More species live on Vitaceae than on other plant families. Beobachtungen zur Biologie zweier Sarbanissa-Arten (Lepidoptera: Noctuidae, Agaristinae) auf der Malayischen Halbinsel Zusammenfassung: Im folgenden Artikel werden Angaben zur Larvalbiolo gie von Sarbanissa transiens (W a l k e r , 1856) (Abb. -

Moths of North Carolina - Early Draft 1
Noctuidae Alypia octomaculata Eight-spotted Forester Moth 20 n=0 • • • • • High Mt. • • • • N 10 • •• • u • • • • m • • • • • • • b • 0 • • e • • • • • r 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 NC counties: 39 • Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec • o • • 20 • f n=9 • = Sighting or Collection Low Mt. High counts of: • • in NC since 2001 F = Not seen since 2001 l 10 3 - Columbus - 2007-03-28 • i 3 - Pender - 2007-04-17 g Status Rank h 2 - Carteret - 2001-04-29 0 NC US NC Global t 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 D Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec a 20 20 t n=23 n=35 e Pd CP s 10 10 0 0 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec Three periods to each month: 1-10 / 11-20 / 21-31 FAMILY: Noctuidae SUBFAMILY: Agaristinae TRIBE: TAXONOMIC_COMMENTS: Nine species are included in this genus from the United States and Argentina, a rather peculiar distribution! North Carolina has but a single species. -
MOTHS of OHIO Field Guide DIVISION of WILDLIFE INTRODUCTION HOW to USE THIS GUIDE Text By: David J
MOTHS OF OHIO field guide DIVISION OF WILDLIFE INTRODUCTION HOW TO USE THIS GUIDE Text by: David J. Horn Ph.D Scientific Name Common Name Moths are one of the most diverse and plentiful Group and Family Description: Featured Species groups of insects in Ohio, and the world. An esti- Secondary images 1 Primary Image mated 160,000 species have thus far been catalogued Secondary images 2 Occurrence worldwide, and about 13,000 species have been Size: when at rest found in North America north of Mexico. We do not Visual Index Ohio Distribution yet have a clear picture of the total number of moth Current Page species in Ohio, as new species are still added annu- Description: Habitat & Host Plant Credit & Copyright ally, but the number of species is certainly over 3,000. Although not as popular as butterflies, moths are far Compared to many groups of animals, our knowledge of moth distribution is very more numerous than their better known kin. There is incomplete. Many areas of the state have not been thoroughly surveyed and in some at least twenty times the number of species of moths counties hardly any species have been documented. Accordingly, the distribution maps in Ohio as there are butterflies. in this booklet have three levels of shading: 1. heavily-shaded means a species record documented by specimen or photograph and confirmed by the Ohio Lepidop- The world of moths is one of extraordinary terists. 2. Intermediate shading indicates that the moth is almost certainly present beauty, fantastic behavior, and outrageous diversity. and could be found at the right season.