Assessing the Environmental Adaptation of Wildlife And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Apples Catalogue 2019
ADAMS PEARMAIN Herefordshire, England 1862 Oct 15 Nov Mar 14 Adams Pearmain is a an old-fashioned late dessert apple, one of the most popular varieties in Victorian England. It has an attractive 'pearmain' shape. This is a fairly dry apple - which is perhaps not regarded as a desirable attribute today. In spite of this it is actually a very enjoyable apple, with a rich aromatic flavour which in apple terms is usually described as Although it had 'shelf appeal' for the Victorian housewife, its autumnal colouring is probably too subdued to compete with the bright young things of the modern supermarket shelves. Perhaps this is part of its appeal; it recalls a bygone era where subtlety of flavour was appreciated - a lovely apple to savour in front of an open fire on a cold winter's day. Tree hardy. Does will in all soils, even clay. AERLIE RED FLESH (Hidden Rose, Mountain Rose) California 1930’s 19 20 20 Cook Oct 20 15 An amazing red fleshed apple, discovered in Aerlie, Oregon, which may be the best of all red fleshed varieties and indeed would be an outstandingly delicious apple no matter what color the flesh is. A choice seedling, Aerlie Red Flesh has a beautiful yellow skin with pale whitish dots, but it is inside that it excels. Deep rose red flesh, juicy, crisp, hard, sugary and richly flavored, ripening late (October) and keeping throughout the winter. The late Conrad Gemmer, an astute observer of apples with 500 varieties in his collection, rated Hidden Rose an outstanding variety of top quality. -

Vurdering Av Fortsatt Kalkingsbehov I Kalkede Innsjøer I Telemark Holmen På Lifjell
RAPPORT L.NR. 6507-2013 Vurdering av fortsatt kalkingsbehov i kalkede innsjøer i Telemark Holmen på Lifjell. Foto: Arne William Hjeltnes William Arne Holmen på Lifjell. Foto: Norsk institutt for vannforskning RAPPORT Hovedkontor Sørlandsavdelingen Østlandsavdelingen Vestlandsavdelingen NIVA Midt-Norge Gaustadalléen 21 Jon Lilletuns vei 3 Sandvikaveien 59 Thormøhlensgate 53 D Høgskoleringen 9 0349 Oslo 4879 Grimstad 2312 Ottestad 5006 Bergen 7034 Trondheim Telefon (47) 22 18 51 00 Telefon (47) 22 18 51 00 Telefon (47) 22 18 51 00 Telefon (47) 22 18 51 00 Telefon (47) 22 18 51 00 Telefax (47) 22 18 52 00 Telefax (47) 37 04 45 13 Telefax (47) 62 57 66 53 Telefax (47) 55 31 22 14 Telefax (47) 73 54 63 87 Internett: www.niva.no Tittel Løpenr. (for bestilling) Dato Vurdering av fortsatt kalkingsbehov i kalkede innsjøer i Telemark 6507-2013 Mars 2013 Prosjektnr. Undernr. Sider Pris 10289 58 Forfatter(e) Fagområde Distribusjon Kari Austnes Sur nedbør Åpen Geografisk område Trykket Telemark NIVA Oppdragsgiver(e) Oppdragsreferanse Direktoratet for naturforvaltning Hanne Hegseth Sammendrag Sur nedbør har blitt kraftig redusert de siste 30 årene. Dette har ført til en gradvis bedring av vannkvaliteten med hensyn til forsuring i innsjøer, spesielt i Sør-Norge. Mange innsjøer i Telemark blir kalket for å motvirke effektene av sur nedbør. Når vannkvaliteten er tilstrekkelig forbedret kan kalkingen opphøre. Formålet med dette prosjektet var å vurdere behovet for fortsatt kalking i 339 kalkede innsjøer i Telemark. En statistisk modell er utviklet for å kunne estimere hvilken vannkvalitet kalkpåvirkede innsjøer ville hatt hvis de ikke var kalket. -

UNDERSTANDING HORSE BEHAVIOR Prepared By: Warren Gill, Professor Doyle G
4-H MEMBER GUIDE Agricultural Extension Service Institute of Agriculture HORSE PROJECT PB1654 UNIT 8 GRADE 12 UUNDERSTANDINGNDERSTANDING HHORSEORSE BBEHAVIOREHAVIOR 1 CONTENTS Introduction 3 Planning Your Project 3 The Basics of Horse Behavior 3 Types of Behavior 4 Horse Senses 4 Horse Communication 10 Domestication & Behavior 11 Mating Behavior 11 Behavior at Foaling Time 13 Feeding Behavior 15 Abnormal Behavior / Vices 18 Questions and Answers about Horses 19 References 19 Exercises 20 Glossary 23 SKILLS AND KNOWLEDGE TO BE ACQUIRED • Improved understanding of why horses behave like horses • Applying basic behavioral knowledge to improve training skills • Learning to prevent and correct behavioral problems • Better ways to manage horses through better understanding of horse motivation OBJECTIVES To help you: • Be more competent in horse-related skills and knowledge • Feel more confident around horses • Understand the applications of basic knowledge to practical problems REQUIREMENTS 1. Make a project plan 2. Complete this manual 3. Work on this project with others, including other 4-H members, 4-H leaders, your 4-H agent and other youth and adults who can assist you in your project. 4. Evaluate your accomplishments cover photo by2 Lindsay German UNDERSTANDING HORSE BEHAVIOR Prepared by: Warren Gill, Professor Doyle G. Meadows, Professor James B. Neel, Professor Animal Science Department The University of Tennessee INTRODUCTION he 4-H Horse Project offers 4-H’ers opportunities for growing and developing interest in horses. This manual should help expand your knowledge about horse behavior, which will help you better under T stand why a horse does what it does. The manual contains information about the basics of horse behavior, horse senses, domestication, mating behavior, ingestive (eating) behavior, foaling-time behavior and how horses learn. -

The Use of Stone and Hunting of Reindeer
ARCHAEOLOGY AND ENVIRONMENT 12 THE USE OF STONE AND HUNTING OF REINDEER By Lena Holm O m University of Umeå ° Ai. ^ Department of Archaeology ARCHAEOLOGY AND ENVIRONMENT 12 Distribution: Department of Archaeology, University of Umeå S-901 87 Umeå, Sweden Lena Holm THE USE OF STONE AND HUNTING OF REINDEER A Study of Stone Tool Manufacture and Hunting of Large Mammals in the Central Scandes c. 6 000 - 1 BC. Akademisk avhandling, som för avläggande av filosofie doktors examen vid universitetet i Umeå kommer att offentligt för svaras i hörsal F, Humanisthuset, Umeå universitet, fredagen den 31 januari 1992 klockan 10.00. Abstract The thesis raises questions concerning prehistoric conditions in a high mountain region in central Scandinavia; it focuses on the human use of stone and on hunting principally of reindeer. An analysis of how the stone material was utilized and an approach to how large mammals were hunt ed result in a synthesis describing one interpretation of how the vast landscape of a region in the central Scandinavian high mountains was used. With this major aim as a base questions were posed concerning the human use of stone resources and possible changes in this use. Preconditions for the occurrence of large mammals as game animals and for hunting are also highlighted. A general perspective is the long time period over which possible changes in the use of stone and hunting of big game, encompassing the Late Mesolithic, Neolithic, Bronze Age and to a certain extent the Early Iron Age. Considering the manufacture of flaked stone tools, debitage in the form of flakes from a dwelling, constitute the base where procurement and technology are essential. -

Gamasid Mites
NATIONAL RESEARCH TOMSK STATE UNIVERSITY BIOLOGICAL INSTITUTE RUSSIAN ACADEMY OF SCIENCE ZOOLOGICAL INSTITUTE M.V. Orlova, M.K. Stanyukovich, O.L. Orlov GAMASID MITES (MESOSTIGMATA: GAMASINA) PARASITIZING BATS (CHIROPTERA: RHINOLOPHIDAE, VESPERTILIONIDAE, MOLOSSIDAE) OF PALAEARCTIC BOREAL ZONE (RUSSIA AND ADJACENT COUNTRIES) Scientific editor Andrey S. Babenko, Doctor of Science, professor, National Research Tomsk State University Tomsk Publishing House of Tomsk State University 2015 UDK 576.89:599.4 BBK E693.36+E083 Orlova M.V., Stanyukovich M.K., Orlov O.L. Gamasid mites (Mesostigmata: Gamasina) associated with bats (Chiroptera: Vespertilionidae, Rhinolophidae, Molossidae) of boreal Palaearctic zone (Russia and adjacent countries) / Scientific editor A.S. Babenko. – Tomsk : Publishing House of Tomsk State University, 2015. – 150 р. ISBN 978-5-94621-523-7 Bat gamasid mites is a highly specialized ectoparasite group which is of great interest due to strong isolation and other unique features of their hosts (the ability to fly, long distance migration, long-term hibernation). The book summarizes the results of almost 60 years of research and is the most complete summary of data on bat gamasid mites taxonomy, biology, ecol- ogy. It contains the first detailed description of bat wintering experience in sev- eral regions of the boreal Palaearctic. The book is addressed to zoologists, ecologists, experts in environmental protection and biodiversity conservation, students and teachers of biology, vet- erinary science and medicine. UDK 576.89:599.4 -

Influence of Parasites on Fitness Parameters of the European Hedgehog (Erinaceus Europaeus)
Influence of parasites on fitness parameters of the European hedgehog (Erinaceus europaeus ) Zur Erlangung des akademischen Grades eines DOKTORS DER NATURWISSENSCHAFTEN (Dr. rer. nat.) Fakultät für Chemie und Biowissenschaften Karlsruher Institut für Technologie (KIT) – Universitätsbereich vorgelegte DISSERTATION von Miriam Pamina Pfäffle aus Heilbronn Dekan: Prof. Dr. Stefan Bräse Referent: Prof. Dr. Horst Taraschewski Korreferent: Prof. Dr. Agustin Estrada-Peña Tag der mündlichen Prüfung: 19.10.2010 For my mother and my sister – the strongest influences in my life “Nose-to-nose with a hedgehog, you get a chance to look into its eyes and glimpse a spark of truly wildlife.” (H UGH WARWICK , 2008) „Madame Michel besitzt die Eleganz des Igels: außen mit Stacheln gepanzert, eine echte Festung, aber ich ahne vage, dass sie innen auf genauso einfache Art raffiniert ist wie die Igel, diese kleinen Tiere, die nur scheinbar träge, entschieden ungesellig und schrecklich elegant sind.“ (M URIEL BARBERY , 2008) Index of contents Index of contents ABSTRACT 13 ZUSAMMENFASSUNG 15 I. INTRODUCTION 17 1. Parasitism 17 2. The European hedgehog ( Erinaceus europaeus LINNAEUS 1758) 19 2.1 Taxonomy and distribution 19 2.2 Ecology 22 2.3 Hedgehog populations 25 2.4 Parasites of the hedgehog 27 2.4.1 Ectoparasites 27 2.4.2 Endoparasites 32 3. Study aims 39 II. MATERIALS , ANIMALS AND METHODS 41 1. The experimental hedgehog population 41 1.1 Hedgehogs 41 1.2 Ticks 43 1.3 Blood sampling 43 1.4 Blood parameters 45 1.5 Regeneration 47 1.6 Climate parameters 47 2. Hedgehog dissections 48 2.1 Hedgehog samples 48 2.2 Biometrical data 48 2.3 Organs 49 2.4 Parasites 50 3. -
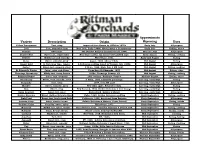
Variety Description Origin Approximate Ripening Uses
Approximate Variety Description Origin Ripening Uses Yellow Transparent Tart, crisp Imported from Russia by USDA in 1870s Early July All-purpose Lodi Tart, somewhat firm New York, Early 1900s. Montgomery x Transparent. Early July Baking, sauce Pristine Sweet-tart PRI (Purdue Rutgers Illinois) release, 1994. Mid-late July All-purpose Dandee Red Sweet-tart, semi-tender New Ohio variety. An improved PaulaRed type. Early August Eating, cooking Redfree Mildly tart and crunchy PRI release, 1981. Early-mid August Eating Sansa Sweet, crunchy, juicy Japan, 1988. Akane x Gala. Mid August Eating Ginger Gold G. Delicious type, tangier G Delicious seedling found in Virginia, late 1960s. Mid August All-purpose Zestar! Sweet-tart, crunchy, juicy U Minn, 1999. State Fair x MN 1691. Mid August Eating, cooking St Edmund's Pippin Juicy, crisp, rich flavor From Bury St Edmunds, 1870. Mid August Eating, cider Chenango Strawberry Mildly tart, berry flavors 1850s, Chenango County, NY Mid August Eating, cooking Summer Rambo Juicy, tart, aromatic 16th century, Rambure, France. Mid-late August Eating, sauce Honeycrisp Sweet, very crunchy, juicy U Minn, 1991. Unknown parentage. Late Aug.-early Sept. Eating Burgundy Tart, crisp 1974, from NY state Late Aug.-early Sept. All-purpose Blondee Sweet, crunchy, juicy New Ohio apple. Related to Gala. Late Aug.-early Sept. Eating Gala Sweet, crisp New Zealand, 1934. Golden Delicious x Cox Orange. Late Aug.-early Sept. Eating Swiss Gourmet Sweet-tart, juicy Switzerland. Golden x Idared. Late Aug.-early Sept. All-purpose Golden Supreme Sweet, Golden Delcious type Idaho, 1960. Golden Delicious seedling Early September Eating, cooking Pink Pearl Sweet-tart, bright pink flesh California, 1944, developed from Surprise Early September All-purpose Autumn Crisp Juicy, slow to brown Golden Delicious x Monroe. -

Environmental Assessment DOI-BLM-ORWA-B050-2018-0016-EA
United States Department of the Interior Bureau of Land Management Burns District Office 28910 Highway 20 West Hines, Oregon 97738 541-589-4400 Phone 541-573-4411 Fax Spay Feasibility and On-Range Behavioral Outcomes Assessment and Warm Springs HMA Population Management Plan Environmental Assessment DOI-BLM-ORWA-B050-2018-0016-EA June 29, 2018 This Page is Intentionally Left Blank Spay Feasibility and On-Range Behavioral Outcomes Assessment and Warm Springs HMA Population Management Plan Environmental Assessment DOI-BLM-ORWA-B050-2018-0016-EA Table of Contents I. INTRODUCTION .........................................................................................................1 A. Background................................................................................................................ 1 B. Purpose and Need for Proposed Action..................................................................... 4 C. Decision to be Made .................................................................................................. 5 D. Conformance with BLM Resource Management Plan(s) .......................................... 6 E. Consistency with Laws, Regulations and Policies..................................................... 7 F. Scoping and Identification of Issues ........................................................................ 12 1. Issues for Analysis .......................................................................................... 13 2. Issues Considered but Eliminated from Detailed Analysis ............................ -

Community Structure of Mites (Acari: Acariformes and Parasitiformes) in Nests of the Semi-Collared Flycatcher (Ficedula Semitorquata) R
International Research Journal of Natural Sciences Vol.3, No.3, pp.48-53, December 2015 ___Published by European Centre for Research Training and Development UK (www.eajournals.org) COMMUNITY STRUCTURE OF MITES (ACARI: ACARIFORMES AND PARASITIFORMES) IN NESTS OF THE SEMI-COLLARED FLYCATCHER (FICEDULA SEMITORQUATA) R. Davidova, V. Vasilev, N. Ali, J. Bakalova Konstantin Preslavsky University of Shumen, 115, Universitetska Str., Shumen, 9700, Bulgaria. ABSTRACT: The aims of the present paper are to establish the specific structure of communities of prostigmatic and mesostigmatic mites in nests of the semi-collared flycatcher (Ficedula semitorquata) and to compare the fauna with the mites in nests of two other European flycatchers. For analysis of community structure of mites were used the indices: prevalence, relative density, mean intensity and dominance. Mite communities are strongly dominated by the species Dermanyssus gallinae and Ornithonyssus sylviarum, which were found with the highest frequency and dominance. The mite communities are characterized by a large number of subrecedent species. KEYWORDS: Acariformes, Parasitiformes, Nest of Bird, Community Structure INTRODUCTION The nests of different species of birds are an example of a fairly unstable and isolated habitat, with its own dependent on it specific fauna which involves different groups of invertebrate animals. One of the components of this fauna which demonstrates particular abundance is the arthropods, and more specifically, the mites. The studies of Parasitiformes show that mesostigmatic mites living in birds' nests vary both in terms of their species affiliation and the structure of their communities [4, 8]. Highly important with respect to veterinary science and medicine are a number of species, such as Ornithonyssus bursa, Ornithonyssus sylviarum, Dermanyssus gallinae harboured by birds, Ornithonyssus bacoti, harboured by rodents, etc. -

Mesostigmata No
16 (1) · 2016 Christian, A. & K. Franke Mesostigmata No. 27 ............................................................................................................................................................................. 1 – 41 Acarological literature .................................................................................................................................................... 1 Publications 2016 ........................................................................................................................................................................................... 1 Publications 2015 ........................................................................................................................................................................................... 9 Publications, additions 2014 ....................................................................................................................................................................... 17 Publications, additions 2013 ....................................................................................................................................................................... 18 Publications, additions 2012 ....................................................................................................................................................................... 20 Publications, additions 2011 ...................................................................................................................................................................... -

Receiver Operating Characteristic (ROC) Methods in Diagnostic Imaging
8/2/2017 Receiver Operating Characteristic (ROC) Methods in Diagnostic Imaging Elizabeth A. Krupinski, PhD Department Radiology & Imaging Sciences Emory University Bit of History • Developed early 1950s based on principles SDT for eval radar operators detecting enemy aircraft & missiles • Contributions from engineering, psychology & mathematics • Lee Lusted introduced medicine 1960s with significant effort on gaining better understanding decision-making • Result of radiology studies after WWII to determine which of 4 radiographic & fluoroscopic techniques better for TB screening • Goal = single imaging technique outperform others • Found intra & inter-observer variation so high impossible determine • Necessary to build systems generate better images so radiologists’ performance could improve (i.e., reduce observer variability) & develop methods evaluate these new systems & assess impact on observer performance Basics • ROC traditionally binary decision task – target/signal (e.g., lesion, disease, missile) present versus target/signal absent, or in case classification rather than detection target/signal belongs to class 1 (e.g., cancer, enemy) or class 2 (e.g., not cancer, friend) • ROC analysis these two conditions must be mutually exclusive 1 8/2/2017 2 x 2 Matrix Decision = Target Decision = Target Present Absent Truth = Target Present True Positive (TP) False Negative (FN) Truth = Target Absent False Positive (FP) True Negative (TN) Common Performance Metrics • Sensitivity = TP/(TP + FN) • Specificity = TN/(TN + FP) • Accuracy = (TP -

Handling of Apple Transport Techniques and Efficiency Vibration, Damage and Bruising Texture, Firmness and Quality
Centre of Excellence AGROPHYSICS for Applied Physics in Sustainable Agriculture Handling of Apple transport techniques and efficiency vibration, damage and bruising texture, firmness and quality Bohdan Dobrzañski, jr. Jacek Rabcewicz Rafa³ Rybczyñski B. Dobrzañski Institute of Agrophysics Polish Academy of Sciences Centre of Excellence AGROPHYSICS for Applied Physics in Sustainable Agriculture Handling of Apple transport techniques and efficiency vibration, damage and bruising texture, firmness and quality Bohdan Dobrzañski, jr. Jacek Rabcewicz Rafa³ Rybczyñski B. Dobrzañski Institute of Agrophysics Polish Academy of Sciences PUBLISHED BY: B. DOBRZAŃSKI INSTITUTE OF AGROPHYSICS OF POLISH ACADEMY OF SCIENCES ACTIVITIES OF WP9 IN THE CENTRE OF EXCELLENCE AGROPHYSICS CONTRACT NO: QLAM-2001-00428 CENTRE OF EXCELLENCE FOR APPLIED PHYSICS IN SUSTAINABLE AGRICULTURE WITH THE th ACRONYM AGROPHYSICS IS FOUNDED UNDER 5 EU FRAMEWORK FOR RESEARCH, TECHNOLOGICAL DEVELOPMENT AND DEMONSTRATION ACTIVITIES GENERAL SUPERVISOR OF THE CENTRE: PROF. DR. RYSZARD T. WALCZAK, MEMBER OF POLISH ACADEMY OF SCIENCES PROJECT COORDINATOR: DR. ENG. ANDRZEJ STĘPNIEWSKI WP9: PHYSICAL METHODS OF EVALUATION OF FRUIT AND VEGETABLE QUALITY LEADER OF WP9: PROF. DR. ENG. BOHDAN DOBRZAŃSKI, JR. REVIEWED BY PROF. DR. ENG. JÓZEF KOWALCZUK TRANSLATED (EXCEPT CHAPTERS: 1, 2, 6-9) BY M.SC. TOMASZ BYLICA THE RESULTS OF STUDY PRESENTED IN THE MONOGRAPH ARE SUPPORTED BY: THE STATE COMMITTEE FOR SCIENTIFIC RESEARCH UNDER GRANT NO. 5 P06F 012 19 AND ORDERED PROJECT NO. PBZ-51-02 RESEARCH INSTITUTE OF POMOLOGY AND FLORICULTURE B. DOBRZAŃSKI INSTITUTE OF AGROPHYSICS OF POLISH ACADEMY OF SCIENCES ©Copyright by BOHDAN DOBRZAŃSKI INSTITUTE OF AGROPHYSICS OF POLISH ACADEMY OF SCIENCES LUBLIN 2006 ISBN 83-89969-55-6 ST 1 EDITION - ISBN 83-89969-55-6 (IN ENGLISH) 180 COPIES, PRINTED SHEETS (16.8) PRINTED ON ACID-FREE PAPER IN POLAND BY: ALF-GRAF, UL.