Evolved Twice Independently Milan Dieris, Daniel Kowatschew & Sigrun I
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Article Evolutionary Dynamics of the OR Gene Repertoire in Teleost Fishes
bioRxiv preprint doi: https://doi.org/10.1101/2021.03.09.434524; this version posted March 10, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Article Evolutionary dynamics of the OR gene repertoire in teleost fishes: evidence of an association with changes in olfactory epithelium shape Maxime Policarpo1, Katherine E Bemis2, James C Tyler3, Cushla J Metcalfe4, Patrick Laurenti5, Jean-Christophe Sandoz1, Sylvie Rétaux6 and Didier Casane*,1,7 1 Université Paris-Saclay, CNRS, IRD, UMR Évolution, Génomes, Comportement et Écologie, 91198, Gif-sur-Yvette, France. 2 NOAA National Systematics Laboratory, National Museum of Natural History, Smithsonian Institution, Washington, D.C. 20560, U.S.A. 3Department of Paleobiology, National Museum of Natural History, Smithsonian Institution, Washington, D.C., 20560, U.S.A. 4 Independent Researcher, PO Box 21, Nambour QLD 4560, Australia. 5 Université de Paris, Laboratoire Interdisciplinaire des Energies de Demain, Paris, France 6 Université Paris-Saclay, CNRS, Institut des Neurosciences Paris-Saclay, 91190, Gif-sur- Yvette, France. 7 Université de Paris, UFR Sciences du Vivant, F-75013 Paris, France. * Corresponding author: e-mail: [email protected]. !1 bioRxiv preprint doi: https://doi.org/10.1101/2021.03.09.434524; this version posted March 10, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Abstract Teleost fishes perceive their environment through a range of sensory modalities, among which olfaction often plays an important role. -

BONY FISHES 602 Bony Fishes
click for previous page BONY FISHES 602 Bony Fishes GENERAL REMARKS by K.E. Carpenter, Old Dominion University, Virginia, USA ony fishes constitute the bulk, by far, of both the diversity and total landings of marine organisms encoun- Btered in fisheries of the Western Central Atlantic.They are found in all macrofaunal marine and estuarine habitats and exhibit a lavish array of adaptations to these environments. This extreme diversity of form and taxa presents an exceptional challenge for identification. There are 30 orders and 269 families of bony fishes presented in this guide, representing all families known from the area. Each order and family presents a unique suite of taxonomic problems and relevant characters. The purpose of this preliminary section on technical terms and guide to orders and families is to serve as an introduction and initial identification guide to this taxonomic diversity. It should also serve as a general reference for those features most commonly used in identification of bony fishes throughout the remaining volumes. However, I cannot begin to introduce the many facets of fish biology relevant to understanding the diversity of fishes in a few pages. For this, the reader is directed to one of the several general texts on fish biology such as the ones by Bond (1996), Moyle and Cech (1996), and Helfman et al.(1997) listed below. A general introduction to the fisheries of bony fishes in this region is given in the introduction to these volumes. Taxonomic details relevant to a specific family are explained under each of the appropriate family sections. The classification of bony fishes continues to transform as our knowledge of their evolutionary relationships improves. -

§4-71-6.5 LIST of CONDITIONALLY APPROVED ANIMALS November
§4-71-6.5 LIST OF CONDITIONALLY APPROVED ANIMALS November 28, 2006 SCIENTIFIC NAME COMMON NAME INVERTEBRATES PHYLUM Annelida CLASS Oligochaeta ORDER Plesiopora FAMILY Tubificidae Tubifex (all species in genus) worm, tubifex PHYLUM Arthropoda CLASS Crustacea ORDER Anostraca FAMILY Artemiidae Artemia (all species in genus) shrimp, brine ORDER Cladocera FAMILY Daphnidae Daphnia (all species in genus) flea, water ORDER Decapoda FAMILY Atelecyclidae Erimacrus isenbeckii crab, horsehair FAMILY Cancridae Cancer antennarius crab, California rock Cancer anthonyi crab, yellowstone Cancer borealis crab, Jonah Cancer magister crab, dungeness Cancer productus crab, rock (red) FAMILY Geryonidae Geryon affinis crab, golden FAMILY Lithodidae Paralithodes camtschatica crab, Alaskan king FAMILY Majidae Chionocetes bairdi crab, snow Chionocetes opilio crab, snow 1 CONDITIONAL ANIMAL LIST §4-71-6.5 SCIENTIFIC NAME COMMON NAME Chionocetes tanneri crab, snow FAMILY Nephropidae Homarus (all species in genus) lobster, true FAMILY Palaemonidae Macrobrachium lar shrimp, freshwater Macrobrachium rosenbergi prawn, giant long-legged FAMILY Palinuridae Jasus (all species in genus) crayfish, saltwater; lobster Panulirus argus lobster, Atlantic spiny Panulirus longipes femoristriga crayfish, saltwater Panulirus pencillatus lobster, spiny FAMILY Portunidae Callinectes sapidus crab, blue Scylla serrata crab, Samoan; serrate, swimming FAMILY Raninidae Ranina ranina crab, spanner; red frog, Hawaiian CLASS Insecta ORDER Coleoptera FAMILY Tenebrionidae Tenebrio molitor mealworm, -

Fish Passage and Injury Risk at a Surface Bypass of a Small-Scale Hydropower Plant
sustainability Article Fish Passage and Injury Risk at a Surface Bypass of a Small-Scale Hydropower Plant Josef Knott, Melanie Mueller, Joachim Pander and Juergen Geist * Aquatic Systems Biology Unit, Department of Ecology and Ecosystem Management, Technical University of Munich, Mühlenweg 22, 85354 Freising, Germany; [email protected] (J.K.); [email protected] (M.M.); [email protected] (J.P.) * Correspondence: [email protected]; Tel.: +49-816-171-3767 Received: 14 October 2019; Accepted: 29 October 2019; Published: 30 October 2019 Abstract: In contrast to the efforts made to develop functioning fishways for upstream migrants, the need for effective downstream migration facilities has long been underestimated. The challenge of developing well-performing bypasses for downstream migrants involves attracting the fish to the entrance and transporting them quickly and unharmed into the tailrace. In this study, the acceptance of different opening sizes of a surface bypass as well as the injuries which fish experience during the passage were examined. Overall bypass acceptance was low compared to the turbine passage. There was no significant difference in the number of downstream moving fish between the small and the large bypass openings. Across all fish species, no immediate mortality was detected. Severe injuries such as amputations or bruises were only rarely detected and at low intensity. Scale losses, tears and hemorrhages in the fins and dermal lesions at the body were the most common injuries, and significant species-specific differences were detected. To increase bypass efficiency, it would likely be useful to offer an alternative bottom bypass in addition to the existing surface bypass. -
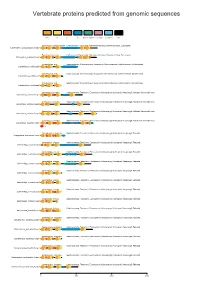
Vertebrate Proteins Predicted from Genomic Sequences
Vertebrate proteins predicted from genomic sequences VWD C8 TIL PTS Mucin2_WxxW F5_F8_type_C FCGBP_N VWC Lethenteron_camtschaticum Cyclostomata; Hyperoartia; Petromyzontiformes; Petromyzontidae; Lethenteron Lethenteron_camtschaticum.0.pep1 Petromyzon_marinus Cyclostomata; Hyperoartia; Petromyzontiformes; Petromyzontidae; Petromyzon Petromyzon_marinus.0.pep1 Callorhinchus_milii Gnathostomata; Chondrichthyes; Holocephali; Chimaeriformes; Callorhinchidae; Callorhinchus Callorhinchus_milii.0.pep1 Callorhinchus_milii Gnathostomata; Chondrichthyes; Holocephali; Chimaeriformes; Callorhinchidae; Callorhinchus Callorhinchus_milii.0.pep2 Callorhinchus_milii Gnathostomata; Chondrichthyes; Holocephali; Chimaeriformes; Callorhinchidae; Callorhinchus Callorhinchus_milii.0.pep3 Lepisosteus_oculatus Gnathostomata; Teleostomi; Euteleostomi; Actinopterygii; Actinopteri; Neopterygii; Holostei; Semionotiformes; Lepisosteus_oculatus.0.pep1 Lepisosteus_oculatus Gnathostomata; Teleostomi; Euteleostomi; Actinopterygii; Actinopteri; Neopterygii; Holostei; Semionotiformes; Lepisosteus_oculatus.0.pep2 Lepisosteus_oculatus Gnathostomata; Teleostomi; Euteleostomi; Actinopterygii; Actinopteri; Neopterygii; Holostei; Semionotiformes; Lepisosteus_oculatus.0.pep3 Lepisosteus_oculatus Gnathostomata; Teleostomi; Euteleostomi; Actinopterygii; Actinopteri; Neopterygii; Holostei; Semionotiformes; Lepisosteus_oculatus.1.pep1 TILa Cynoglossus_semilaevis Gnathostomata; Teleostomi; Euteleostomi; Actinopterygii; Actinopteri; Neopterygii; Teleostei; Cynoglossus_semilaevis.1.pep1 -

Copyrighted Material
Index INDEX Note: page numbers in italics refer to fi gures, those in bold refer to tables and boxes. abducens nerve 55 activity cycles 499–522 inhibition 485 absorption effi ciency 72 annual patterns 515, 516, 517–22 interactions 485–6 abyssal zone 393 circadian rhythms 505 prey 445 Acanthaster planci (Crown-of-Thorns Starfi sh) diel patterns 499, 500, 501–2, 503–4, reduction 484 579 504–7 aggressive mimicry 428, 432–3 Acanthocybium (Wahoo) 15 light-induced 499, 500, 501–2, 503–4, aggressive resemblance 425–6 Acanthodii 178, 179 505 aglomerular 52 Acanthomorpha 284–8, 289 lunar patterns 507–9 agnathans Acanthopterygii 291–325 seasonal 509–15 gills 59, 60 Atherinomorpha 293–6 semilunar patterns 507–9 osmoregulation 101, 102 characteristics 291–2 supra-annual patterns 515, 516, 517–22 phylogeny 202 distribution 349, 350 tidal patterns 506–7 ventilation 59, 60 jaws 291 see also migration see also hagfi shes; lampreys Mugilomorpha 292–3, 294 adaptive response 106 agnathous fi shes see jawless fi shes pelagic 405 adaptive zones 534 agonistic interactions 83–4, 485–8 Percomorpha 296–325 adenohypophysis 91, 92 chemically mediated 484 pharyngeal jaws 291 adenosine triphosphate (ATP) 57 sound production 461–2 phylogeny 292, 293, 294 adipose fi n 35 visual 479 spines 449, 450 adrenocorticotropic hormone (ACTH) 92 agricultural chemicals 605 Acanthothoraciformes 177 adrianichthyids 295 air breathing 60, 61–2, 62–4 acanthurids 318–19 adult fi shes 153, 154, 155–7 ammonia production 64, 100–1 Acanthuroidei 12, 318–19 death 156–7 amphibious 60 Acanthurus bahianus -

The Etyfish Project © Christopher Scharpf and Kenneth J
CYPRINODONTIFORMES (part 3) · 1 The ETYFish Project © Christopher Scharpf and Kenneth J. Lazara COMMENTS: v. 3.0 - 13 Nov. 2020 Order CYPRINODONTIFORMES (part 3 of 4) Suborder CYPRINODONTOIDEI Family PANTANODONTIDAE Spine Killifishes Pantanodon Myers 1955 pan(tos), all; ano-, without; odon, tooth, referring to lack of teeth in P. podoxys (=stuhlmanni) Pantanodon madagascariensis (Arnoult 1963) -ensis, suffix denoting place: Madagascar, where it is endemic [extinct due to habitat loss] Pantanodon stuhlmanni (Ahl 1924) in honor of Franz Ludwig Stuhlmann (1863-1928), German Colonial Service, who, with Emin Pascha, led the German East Africa Expedition (1889-1892), during which type was collected Family CYPRINODONTIDAE Pupfishes 10 genera · 112 species/subspecies Subfamily Cubanichthyinae Island Pupfishes Cubanichthys Hubbs 1926 Cuba, where genus was thought to be endemic until generic placement of C. pengelleyi; ichthys, fish Cubanichthys cubensis (Eigenmann 1903) -ensis, suffix denoting place: Cuba, where it is endemic (including mainland and Isla de la Juventud, or Isle of Pines) Cubanichthys pengelleyi (Fowler 1939) in honor of Jamaican physician and medical officer Charles Edward Pengelley (1888-1966), who “obtained” type specimens and “sent interesting details of his experience with them as aquarium fishes” Yssolebias Huber 2012 yssos, javelin, referring to elongate and narrow dorsal and anal fins with sharp borders; lebias, Greek name for a kind of small fish, first applied to killifishes (“Les Lebias”) by Cuvier (1816) and now a -

Analysis of Genetic Diversity of Leuciscus Leuciscus Baicalensis Using Novel Microsatellite Markers with Cross-Species Transferability
Analysis of genetic diversity of Leuciscus leuciscus baicalensis using novel microsatellite markers with cross-species transferability D.J. Lei1, G. Zhao1, P. Xie1, Y. Li1, H. Yuan1, M. Zou1, J.G. Niu2 and X.F. Ma1 1College of Fisheries, Huazhong Agricultural University, Wuhan, China 2Xinjiang Fisheries Research Institute, Urumqi, China Corresponding author: X.F. Ma E-mail: [email protected] Genet. Mol. Res. 16 (2): gmr16029376 Received September 23, 2016 Accepted March 8, 2017 Published May 4, 2017 DOI http://dx.doi.org/10.4238/gmr16029376 Copyright © 2017 The Authors. This is an open-access article distributed under the terms of the Creative Commons Attribution ShareAlike (CC BY-SA) 4.0 License. ABSTRACT. We used next-generation sequencing technology to characterize 19 genomic simple sequence repeat (SSR) markers and 11 expressed sequence tag (EST) SSR markers from Leuciscus leuciscus baicalensis, a small freshwater fish that is widely distributed in Xinjiang, China. Primers were used to test for polymorphisms in three L. leuciscus baicalensis populations in Xinjiang. There were 4-27 (average 11.3) alleles (NA), the expected heterozygosity (HE) was 0.36-0.94 (average 0.75 ± 0.14), the observed heterozygosity (HO) was 0.37-1.00 (average 0.68 ± 0.18), and the polymorphism information content (PIC) was 0.31-0.93 (average 0.71). The averages of HE and PIC for the EST-SSR markers were slightly lower than for the genomic SSR markers. Genetic analysis of the three populations showed similar results for PIC, HE, and NA. Amplifications were performed in nine other species; the top three transferability values were for Rutilus lacustris (80%), Leuciscus idus (76.7%), and Phoxinus ujmonensis (63.3%), with the following average values: PIC (0.56, 4.46, and 0.52); NA (0.40, 3.00, and 0.32); and HO (0.44, 2.74, and 0.22), respectively. -

Download Document
Downloaded from UvA-DARE, the institutional repository of the University of Amsterdam (UvA) http://dare.uva.nl/document/197409 File ID 197409 Filename Chapter 5 Geographic variation in Poecilia Bloch and Schneider, 1801 (Teleostei: Poeciliidae), with descriptions of three new species and lectotypes for P. dovii Günther, 1866 and for P. vandepolli van Lidth de Jeude, 1887 SOURCE (OR PART OF THE FOLLOWING SOURCE): Type Dissertation Title From the Amazonriver to the Amazon molly and back again Author F. Poeser Faculty Faculty of Science Year 2003 Pages 180 ISBN 9076894329 FULL BIBLIOGRAPHIC DETAILS: http://dare.uva.nl/record/115955 Copyright It is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), other than for strictly personal, individual use. UvA-DARE is a service provided by the library of the University of Amsterdam (http://dare.uva.nl) 44 From the Amazon river to the Amazon molly and back again: Chapter 5 Geographic variation in Poecilia Bloch and Schneider, 1801 (Teleostei: Poeciliidae), with descriptions of three new species and lectotypes for P. dovii Giinther, 1866 and for P. vandepolli van Lidth de Jeude, 1887 Fred. N. Poeser Institute for Systematics and Population Biology, Department of Ichthyology, University of Amsterdam P.O. Box 94766, 1090 GT Amsterdam, The Netherlands Abstract The South American species with the vernacular name "mollies" are analyzed and three new species of the genus Poecilia are described and figured, viz., P. boesemani n. sp. from Trinidad, P. koperi n. sp. from Venezuela and Colombia, and P. -

Updated Checklist of Marine Fishes (Chordata: Craniata) from Portugal and the Proposed Extension of the Portuguese Continental Shelf
European Journal of Taxonomy 73: 1-73 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.73 www.europeanjournaloftaxonomy.eu 2014 · Carneiro M. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:9A5F217D-8E7B-448A-9CAB-2CCC9CC6F857 Updated checklist of marine fishes (Chordata: Craniata) from Portugal and the proposed extension of the Portuguese continental shelf Miguel CARNEIRO1,5, Rogélia MARTINS2,6, Monica LANDI*,3,7 & Filipe O. COSTA4,8 1,2 DIV-RP (Modelling and Management Fishery Resources Division), Instituto Português do Mar e da Atmosfera, Av. Brasilia 1449-006 Lisboa, Portugal. E-mail: [email protected], [email protected] 3,4 CBMA (Centre of Molecular and Environmental Biology), Department of Biology, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: [email protected], [email protected] * corresponding author: [email protected] 5 urn:lsid:zoobank.org:author:90A98A50-327E-4648-9DCE-75709C7A2472 6 urn:lsid:zoobank.org:author:1EB6DE00-9E91-407C-B7C4-34F31F29FD88 7 urn:lsid:zoobank.org:author:6D3AC760-77F2-4CFA-B5C7-665CB07F4CEB 8 urn:lsid:zoobank.org:author:48E53CF3-71C8-403C-BECD-10B20B3C15B4 Abstract. The study of the Portuguese marine ichthyofauna has a long historical tradition, rooted back in the 18th Century. Here we present an annotated checklist of the marine fishes from Portuguese waters, including the area encompassed by the proposed extension of the Portuguese continental shelf and the Economic Exclusive Zone (EEZ). The list is based on historical literature records and taxon occurrence data obtained from natural history collections, together with new revisions and occurrences. -

Transcriptome Sequencing and Analysis of Wild Amur Ide (Leuciscus Waleckii) Inhabiting an Extreme Alkaline-Saline Lake Reveals Insights Into Stress Adaptation
Transcriptome Sequencing and Analysis of Wild Amur Ide (Leuciscus waleckii) Inhabiting an Extreme Alkaline- Saline Lake Reveals Insights into Stress Adaptation Jian Xu1, Peifeng Ji1, Baosen Wang1,2, Lan Zhao1, Jian Wang1, Zixia Zhao1, Yan Zhang1, Jiongtang Li1, Peng Xu1*, Xiaowen Sun1* 1 Centre for Applied Aquatic Genomics, Chinese Academy of Fishery Sciences, Beijing, China, 2 College of Life Sciences, Tianjin Normal University, Tianjin, China Abstract Background: Amur ide (Leuciscus waleckii) is an economically and ecologically important species in Northern Asia. The Dali Nor population inhabiting Dali Nor Lake, a typical saline-alkaline lake in Inner Mongolia, is well-known for its adaptation to extremely high alkalinity. Genome information is needed for conservation and aquaculture purposes, as well as to gain further understanding into the genetics of stress tolerance. The objective of the study is to sequence the transcriptome and obtain a well-assembled transcriptome of Amur ide. Results: The transcriptome of Amur ide was sequenced using the Illumina platform and assembled into 53,632 cDNA contigs, with an average length of 647 bp and a N50 length of 1,094 bp. A total of 19,338 unique proteins were identified, and gene ontology and KEGG (Kyoto Encyclopedia of Genes and Genomes) analyses classified all contigs into functional categories. Open Reading Frames (ORFs) were detected from 34,888 (65.1%) of contigs with an average length of 577 bp, while 9,638 full-length cDNAs were identified. Comparative analyses revealed that 31,790 (59.3%) contigs have a significant similarity to zebrafish proteins, and 27,096 (50.5%), 27,524 (51.3%) and 27,996 (52.2%) to teraodon, medaka and three- spined stickleback proteins, respectively. -

The Reproductive Cycle of Female Ballan Wrasse Labrus Bergylta in High Latitude, Temperate Waters
Journal of Fish Biology (2010) doi:10.1111/j.1095-8649.2010.02691.x, available online at www.interscience.wiley.com The reproductive cycle of female Ballan wrasse Labrus bergylta in high latitude, temperate waters S. Muncaster*†‡§, E. Andersson*, O. S. Kjesbu*, G. L. Taranger*, A. B. Skiftesvik† and B. Norberg† *Institute of Marine Research, P.O. Box 187, Nordnes, N-5817 Bergen, Norway, †Institute of Marine Research, Austevoll Research Station, N-5392 Storebø, Norway and ‡Department of Biology, University of Bergen, N-5020 Bergen, Norway (Received 13 August 2009, Accepted 11 April 2010) This 2 year study examined the reproductive cycle of wild female Ballan wrasse Labrus bergylta in western Norway as a precursor to captive breeding trials. Light microscopy of ovarian histol- ogy was used to stage gonad maturity and enzyme-linked immuno-absorbent assay (ELISA) to measure plasma concentrations of the sex steroids testosterone (T) and 17β-oestradiol (E2). Ovar- ian recrudescence began in late autumn to early winter with the growth of previtellogenic oocytes and the formation of cortical alveoli. Vitellogenic oocytes developed from January to June and ovaries containing postovulatory follicles (POF) were present between May and June. These POF occurred simultaneously among other late maturity stage oocytes. Plasma steroid concentration and organo-somatic indices increased over winter and spring. Maximal (mean ± s.e.) values of −1 −1 plasma T (0·95 ± 0·26 ng ml ), E2 (1·75 ± 0·43 ng ml ) and gonado-somatic index (IG;10·71 ± 0·81) occurred in April and May and decreased greatly in July when only postspawned fish with atretic ovaries occurred.