Urinary Tract Innervation the Urinary Bladder and Urethra Require Innervation to Function Effectively
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

St. Lawrence School Subject
St. Lawrence School Subject - Science Class - 4 Chapter - 3 Human Body : Digestive and Excetory System ( Part - 1 ) Learn about * Digestive system * Excretory system * Healthy eating habits Digestive System The process by which food is broken down into a simpler form so that it can be easily taken in or absorbed by our body is called digestion. Many organs work together and help in the process of digestion. The mouth, food pipe, stomach, small and large intestine, liver, rectum, and anus are the main organs of the digestive system. Let us learn about them. Mouth Digestion starts in the mouth. The teeth help to break down and chew food. The chewed food then mixes with a liquid, called saliva, produced in our mouth. It makes the food softer and easier to swallow. The tongue helps in the proper mixing of saliva with the food. Food pipe The food pipe ( oesophagus ) passes the food from the mouth to the stomach. Stomach Inside the stomach, the food is broken down further into smaller pieces by churning and with the help of chemicals called digestive juices. Small intestine From the small intestine, the undigested food passes into the large intestine. The large intestine is a shorter but wider, tube - like structure, which collects the indigestible food from the small intestine. The large intestine absorbs water from this undigested food and forms waste products called faeces. Rectum Rectum is the final part of the large intestine. Faeces are stored in the rectum for a short time before being passed out through anus. Anus Faeces are removed from the body through the anus. -

Urinary Bladder – Proteinaceous Plug
Urinary bladder – Proteinaceous Plug Figure Legend: Figure 1 An eosinophilic amorphous proteinaceous plug in the bladder lumen from a male B6C3F1 mouse in a chronic study. Figure 2 A proteinaceous plug associated with other flocculent, eosinophilic material, from a male F344/N rat in an acute study. Comment: Proteinaceous plugs are commonly noted as a postmortem change resulting from an agonal secretion of accessory sex gland fluids during euthanasia. Proteinaceous plugs vary in size but can be large, filling the urinary bladder (Figure 1 and Figure 2). Microscopically, the plug is composed of a mixture of an amorphous eosinophilic material, sometimes containing desquamated epithelial cells and spermatozoa. Proteinaceous plugs by themselves have no toxicologic importance and are not precursors of calculi. Plugs may be seen with obstructive syndromes associated with bacterial inflammation. They must be differentiated from calculi. Recommendation: Proteinaceous plugs occurring alone and not associated with any pathologic lesions should be recognized as an artifact and should not be diagnosed. Occasionally, proteinaceous plugs are recognized grossly, and the pathologist should use his or her judgment to correlate the gross lesion to an artifactual proteinaceous plug. 1 Urinary bladder – Proteinaceous Plug References: Gaillard ET. 1999. Ureter, urinary bladder and urethra. In: Pathology of the Mouse: Reference and Atlas (Maronpot RR, Boorman GA, Gaul BW, eds). Cache River Press, Vienna, IL, 235– 258. Abstract: http://www.cacheriverpress.com/books/pathmouse.htm Hard GC, Alden CL, Bruner RH, Frith CH, Lewis RM, Owen RA, Krieg K, Durchfeld-Meyer B. 1999. Non-proliferative lesions of the kidney and lower urinary tract in rats. -

Vocabulario De Morfoloxía, Anatomía E Citoloxía Veterinaria
Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) Servizo de Normalización Lingüística Universidade de Santiago de Compostela COLECCIÓN VOCABULARIOS TEMÁTICOS N.º 4 SERVIZO DE NORMALIZACIÓN LINGÜÍSTICA Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) 2008 UNIVERSIDADE DE SANTIAGO DE COMPOSTELA VOCABULARIO de morfoloxía, anatomía e citoloxía veterinaria : (galego-español- inglés) / coordinador Xusto A. Rodríguez Río, Servizo de Normalización Lingüística ; autores Matilde Lombardero Fernández ... [et al.]. – Santiago de Compostela : Universidade de Santiago de Compostela, Servizo de Publicacións e Intercambio Científico, 2008. – 369 p. ; 21 cm. – (Vocabularios temáticos ; 4). - D.L. C 2458-2008. – ISBN 978-84-9887-018-3 1.Medicina �������������������������������������������������������������������������veterinaria-Diccionarios�������������������������������������������������. 2.Galego (Lingua)-Glosarios, vocabularios, etc. políglotas. I.Lombardero Fernández, Matilde. II.Rodríguez Rio, Xusto A. coord. III. Universidade de Santiago de Compostela. Servizo de Normalización Lingüística, coord. IV.Universidade de Santiago de Compostela. Servizo de Publicacións e Intercambio Científico, ed. V.Serie. 591.4(038)=699=60=20 Coordinador Xusto A. Rodríguez Río (Área de Terminoloxía. Servizo de Normalización Lingüística. Universidade de Santiago de Compostela) Autoras/res Matilde Lombardero Fernández (doutora en Veterinaria e profesora do Departamento de Anatomía e Produción Animal. -

1535190852 9 Medical Terminology Urinary.Pdf
Syrian Private University Medical Faculty Medical Terminology M.A.Kubtan , MD – FRCS Lecture 9 M.A.Kubtan Objectives After studying this chapter, you will be able to: •Name the parts of the urinary system and discuss the function of each part •Define combining forms used in building words that relate to the urinary system •Identify the meaning of related abbreviations •Name the common diagnoses, clinical procedures, and laboratory tests used in treating disorders of the urinary system M.A.Kubtan 2 Objectives Part 2 •List and define the major pathological conditions of the urinary system •Explain the meaning of surgical terms related to the urinary system •Recognize common pharmacological agents used in treating the urinary system M.A.Kubtan 3 Structure and Function The Urinary System Bladder Kidneys •Also called the excretory system •Maintains water Urinary System balance •Removes waste products from the Urethra Ureters blood by excreting them in the urine Meatus M.A.Kubtan 4 Kidneys Kidneys The kidneys are bean-shaped organs located in the retroperitoneal portion of the abdominal cavity on either side of the vertebral column. Two Primary Functions •To form urine for excretion •To retain essential substances the body needs in the process called reabsorption M.A.Kubtan 5 Parts of the Kidney Kidneys filter about 1700 kidney liters of blood daily in the average adult. medulla Parts of the kidneys •Cortex hilum -outer protective portion •Medulla -inner soft portion •Hilum -a depression located in the middle of the concave side of the kidney where blood vessels, nerves, and the ureters enter and cortex exit the kidneys M.A.Kubtan 6 Urine Production Urine is produced by filtration of: •water •sugar •creatine •salts •urea •uric acid Each kidney contains more than 1 million nephrons which are the functional units of the kidneys. -

Urology & Incontinence
Urology & Incontinence - Glossary of Terms Anti-reflux Refers to a tube or collapsible material within a urine collection device to help prevent urine from reentering the tubing. Applicator collar Found on some Hollister male external catheters, a plastic guide with notches for the thumb and forefinger to assist proper placement against the tip of the penis. Aseptic intermittent catheterization The process of performing intermittent catheterization using sterile equipment and inserting the catheter in a sterile way. This would include a sterile ready-to-use product that can be inserted with gloves using a no-touch technique (e.g., the Advance Plus intermittent catheter or a VaPro hydrophilic catheter). Benzalkonium chloride (BZK) An antimicrobial solution used for cleansing the urethral opening prior to inserting an intermittent catheter. Does not stain skin or clothing. Bladder A collapsible balloon-like muscular organ that lies in the pelvis and functions to store and expel urine. Bladder catheterization A procedure in which a catheter is passed through the urethra or stoma into the bladder, usually for the purpose of draining urine. Bladder control The ability to control urination. Bladder diary A printed or electronic form to keep track of when one urinates or leaks urine. Catheter (urinary) A special type of hollow tube inserted through the urethra or a stoma to the bladder to withdraw urine or instill medication. Catheterization The process of inserting a tube into the bladder to drain urine. Clean intermittent catheterization The process of emptying the bladder using a clean intermittent catheter. It involves inserting and removing a catheter, typically several times a day. -
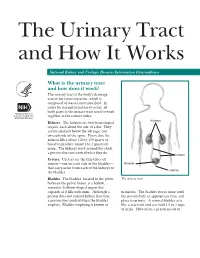
The Urinary Tract and How It Works
The Urinary Tract and How It Works National Kidney and Urologic Diseases Information Clearinghouse What is the urinary tract and how does it work? The urinary tract is the body’s drainage system for removing urine, which is composed of wastes and extra fluid. In order for normal urination to occur, all body parts in the urinary tract need to work together in the correct order. Kidneys Kidneys. The kidneys are two bean-shaped organs, each about the size of a fist. They are located just below the rib cage, one on each side of the spine. Every day, the kidneys filter about 120 to 150 quarts of blood to produce about 1 to 2 quarts of urine. The kidneys work around the clock; a person does not control what they do. Ureters Ureters. Ureters are the thin tubes of muscle—one on each side of the bladder— Bladder that carry urine from each of the kidneys to Urethra the bladder. Bladder. The bladder, located in the pelvis The urinary tract between the pelvic bones, is a hollow, muscular, balloon-shaped organ that expands as it fills with urine. Although a urination. The bladder stores urine until person does not control kidney function, the person finds an appropriate time and a person does control when the bladder place to urinate. A normal bladder acts empties. Bladder emptying is known as like a reservoir and can hold 1.5 to 2 cups of urine. How often a person needs to urinate depends on how quickly the kidneys Why is the urinary tract produce the urine that fills the bladder. -

35-40 Institute of Normal Anatomy
35 Lymphology 28 (1995) 35-40 Institute of Normal Anatomy (PP,AT), Institute of Histology and General Embryology (CM), and Department of Human Pathology (RS), University of Pavia, Italy ABSTRACT light and electron microscopy (2,3). To clarify vesical lymph drainage, we now examined the After endoscopic transurethral biopsies of fine structure of small lymphatic vessels (SL V) normal human urinary bladder, an extensive and their distribution in the normal human network of small initial lymphatic vessels was urinary bladder. Particular attention was depicted by means of light and electron directed to the structure and composition of microscopy. Using light microscopy, lymphatic the connective matrix that surrounds the vessels were seen in the mucosa and lymphatic vessels in the different regions of submucosa and formed a complex network in the bladder wall. the detrusor muscular coat. These lymphatics were characterized by an irregular and MATERIALS AND METHODS attenuated wall and increased in number and size from the superficial to the deeper region of Ten male subjects (40 to 60 years of age) the bladder. Ultrastructurally, the lymphatic who had symptoms of bladder outlet wall was characterized by endothelial cells obstruction were utilized for this investigation. joined together end-to-end or by complicated Two endoscopic transurethral biopsies from interdigitations. Often intercellular channels the lateral wall of the normal urinary bladder and gaps between two contiguous endothelial were performed under a constant bladder cells were present. A broad network of elastic distention after introduction of physiological and collagen fibers joined the lymphatic saline at 50 cm H20 constant pressure. The endothelial wall to the neighboring connective biopsy trocar was regulated to obtain vesical tissue. -

15-1040-Junu Oh-Neuronal.Key
Neuronal Control of the Bladder Seung-June Oh, MD Department of urology, Seoul National University Hospital Seoul National University College of Medicine Contents Relevant end organs and nervous system Reflex pathways Implication in the sacral neuromodulation Urinary bladder ! body: detrusor ! trigone and bladder neck Urethral sphincters B Preprostatic S Smooth M. Sphincter Passive Prostatic S Skeletal M. Sphincter P Prostatic SS P-M Striated Sphincter Membraneous SS Periurethral Striated M. Pubococcygeous Spinal cord ! S2–S4 spinal cord ! primary parasympathetic micturition center ! bladder and distal urethral sphincter ! T11-L2 spinal cord ! sympathetic outflow ! bladder and proximal urethral sphincter Peripheral innervation ! The lower urinary tract is innervated by 3 principal sets of peripheral nerves: ! parasympathetic -pelvic n. ! sympathetic-hypogastric n. ! somatic nervous systems –pudendal n. ! Parasympathetic and sympathetic nervous systems form pelvic plexus at the lateral side of the rectum before reaching bladder and sphincter Sympathetic & parasympathetic systems ! Sympathetic pathways ! originate from the T11-L2 (sympathetic nucleus; intermediolateral column of gray matter) ! inhibiting the bladder body and excite the bladder base and proximal urethral sphincter ! Parasympathetic nerves ! emerge from the S2-4 (parasympathetic nucleus; intermediolateral column of gray matter) ! exciting the bladder and relax the urethra Sacral somatic system !emerge from the S2-4 (Onuf’s nucleus; ventral horn) !form pudendal nerve, providing -

Clinical and Functional Anatomy of the Urethral Sphincter
Review Article International Neurourology Journal Int Neurourol J 2012;16:102-106 http://dx.doi.org/10.5213/inj.2012.16.3.102 pISSN 2093-4777 · eISSN 2093-6931 INJ Clinical and Functional Anatomy of the Urethral Sphincter Junyang Jung, Hyo Kwang Ahn, Youngbuhm Huh Department of Anatomy, Kyung Hee University School of Medicine, Seoul, Korea Continence and micturition involve urethral closure. Especially, insufficient strength of the pelvic floor muscles including the urethral sphincter muscles causes urinary incontinence (UI). Thus, it is most important to understand the main mechanism causing UI and the relationship of UI with the urethral sphincter. Functionally and anatomically, the urethral sphincter is made up of the internal and the external sphincter. We highlight the basic and clinical anatomy of the internal and the external sphinc- ter and their clinical meaning. Understanding these relationships may provide a novel view in identifying the main mechanism causing UI and surgical techniques for UI. Keywords: Urethral sphincters; Pudendal nerve; Autonomic nervous system; Urinary incontinence; Urination INTRODUCTION tomical damage to the ligaments, facial support, and pelvic floor musculature, including the levator ani [8]. The pudendal nerve The urethral sphincter is crucial for the maintenance of urinary innervating the EUS is susceptible to injury during vaginal birth continence [1,2]. The urethral sphincter refers to one of the fol- because it travels between the sacrospinous and sacrotuberous lowing muscles [3]: 1) the internal urethral sphincter (IUS), ligaments [9]. In this article, we discuss the basic and clinical which consists of smooth muscle and is continuous with the anatomy of the urethral sphincter and the relationship between detrusor muscle and under involuntary control, and 2) the ex- the urethral sphincter and UI. -
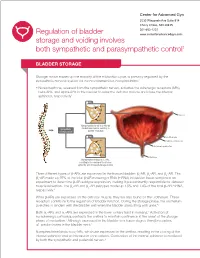
Regulation of Bladder Storage and Voiding Involves Both Sympathetic
Center for Advanced Gyn 5530 Wisconsin Ave Suite 914 Chevy Chase, MD 20815 301-652-1231 Regulation of bladder www.centerforadvancedgyn.com storage and voiding involves both sympathetic and parasympathetic control1 BLADDER STORAGE Storage, which makes up the majority of the micturition cycle, is primarily regulated by the sympathetic nervous system via the neurotransmitter, norepinephrine.2 • Norepinephrine, released from the sympathetic nerves, activates the adrenergic receptors (ARs), beta-ARs, and alpha-ARs in the bladder to relax the detrusor muscle and close the internal sphincter, respectively2 β-ARs Norepinephrine Ureter β-AR Norepinephrine binds to β-ARs on Sympathetic the detrusor muscle, resulting in nervous system bladder relaxation α-ARs Urothelium -AR α1 Detrusor muscle Internal sphincter Norepinephrine Urethra Norepinephrine binds to α1-ARs, resulting in the closing of the internal sphincter and increased storage of urine Three different types of b-ARs are expressed in the human bladder: b1-AR, b2-AR, and b3-AR. The b3-AR made up 97% of the total b-AR messenger RNA (mRNA) in bladder tissue samples in an experiment to determine b-AR subtype expression, making it predominantly responsible for detrusor muscle relaxation. The b1-AR and b2-AR subtypes made up 1.5% and 1.4% of the total b-AR mRNA, respectively.3 While b-ARs are expressed on the detrusor muscle, they are also found on the urothelium. These receptors contribute to the regulation of bladder function. During the storage phase, the urothelium stretches in tandem with the bladder wall when the bladder starts filling with urine.4,5 6 Both a1-ARs and a2-ARs are expressed in the lower urinary tract in humans. -

Urinary System
OUTLINE 27.1 General Structure and Functions of the Urinary System 818 27.2 Kidneys 820 27 27.2a Gross and Sectional Anatomy of the Kidney 820 27.2b Blood Supply to the Kidney 821 27.2c Nephrons 824 27.2d How Tubular Fluid Becomes Urine 828 27.2e Juxtaglomerular Apparatus 828 Urinary 27.2f Innervation of the Kidney 828 27.3 Urinary Tract 829 27.3a Ureters 829 27.3b Urinary Bladder 830 System 27.3c Urethra 833 27.4 Aging and the Urinary System 834 27.5 Development of the Urinary System 835 27.5a Kidney and Ureter Development 835 27.5b Urinary Bladder and Urethra Development 835 MODULE 13: URINARY SYSTEM mck78097_ch27_817-841.indd 817 2/25/11 2:24 PM 818 Chapter Twenty-Seven Urinary System n the course of carrying out their specific functions, the cells Besides removing waste products from the bloodstream, the uri- I of all body systems produce waste products, and these waste nary system performs many other functions, including the following: products end up in the bloodstream. In this case, the bloodstream is ■ Storage of urine. Urine is produced continuously, but analogous to a river that supplies drinking water to a nearby town. it would be quite inconvenient if we were constantly The river water may become polluted with sediment, animal waste, excreting urine. The urinary bladder is an expandable, and motorboat fuel—but the town has a water treatment plant that muscular sac that can store as much as 1 liter of urine. removes these waste products and makes the water safe to drink. -

Nerve Disease and Bladder Control
Nerve Disease and Bladder Control National Kidney and Urologic Diseases Information Clearinghouse For the urinary system to do its job, muscles and nerves must work together to hold Brain urine in the bladder and then release it at the right time. Nerves carry messages from NATIONAL the bladder to the brain to let it know when INSTITUTES the bladder is full. They also carry messages OF HEALTH Central nervous from the brain to the bladder, telling muscles system (brain either to tighten or release. A nerve prob and spinal cord) lem might affect your bladder control if the nerves that are supposed to carry messages Spinal cord between the brain and the bladder do not work properly. Nerve signals U.S. Department to bladder of Health and Bladder and sphincter Human Services What bladder control muscles problems does nerve damage cause? Nerves that work poorly can lead to three Urethra different kinds of bladder control problems. Overactive bladder. Damaged nerves may Sphincter muscles send signals to the bladder at the wrong time, causing its muscles to squeeze with Nerves carry signals from the brain to the bladder out warning. The symptoms of overactive and sphincter. bladder include • urinary frequency—defined as urination eight or more times a day or two or nerves to the sphincter muscles are dam more times at night aged, the muscles may become loose and allow leakage or stay tight when you are • urinary urgency—the sudden, strong trying to release urine. need to urinate immediately Urine retention. For some people, nerve • urge incontinence—leakage of urine damage means their bladder muscles do that follows a sudden, strong urge to not get the message that it is time to release urinate urine or are too weak to completely empty Poor control of sphincter muscles.