Modelling a Small Freshwater Food Web from an Experimental Data
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Phylogenetic Relationships Within the Speciose Family Characidae
Oliveira et al. BMC Evolutionary Biology 2011, 11:275 http://www.biomedcentral.com/1471-2148/11/275 RESEARCH ARTICLE Open Access Phylogenetic relationships within the speciose family Characidae (Teleostei: Ostariophysi: Characiformes) based on multilocus analysis and extensive ingroup sampling Claudio Oliveira1*, Gleisy S Avelino1, Kelly T Abe1, Tatiane C Mariguela1, Ricardo C Benine1, Guillermo Ortí2, Richard P Vari3 and Ricardo M Corrêa e Castro4 Abstract Background: With nearly 1,100 species, the fish family Characidae represents more than half of the species of Characiformes, and is a key component of Neotropical freshwater ecosystems. The composition, phylogeny, and classification of Characidae is currently uncertain, despite significant efforts based on analysis of morphological and molecular data. No consensus about the monophyly of this group or its position within the order Characiformes has been reached, challenged by the fact that many key studies to date have non-overlapping taxonomic representation and focus only on subsets of this diversity. Results: In the present study we propose a new definition of the family Characidae and a hypothesis of relationships for the Characiformes based on phylogenetic analysis of DNA sequences of two mitochondrial and three nuclear genes (4,680 base pairs). The sequences were obtained from 211 samples representing 166 genera distributed among all 18 recognized families in the order Characiformes, all 14 recognized subfamilies in the Characidae, plus 56 of the genera so far considered incertae sedis in the Characidae. The phylogeny obtained is robust, with most lineages significantly supported by posterior probabilities in Bayesian analysis, and high bootstrap values from maximum likelihood and parsimony analyses. -

Someone Like Me: Size-Assortative Pairing and Mating in an Amazonian Fish, Sailfin Tetra Crenuchus Spilurus
RESEARCH ARTICLE Someone like me: Size-assortative pairing and mating in an Amazonian fish, sailfin tetra Crenuchus spilurus 1,2 1 1 Elio de Almeida BorghezanID *, Kalebe da Silva Pinto , Jansen Zuanon , Tiago Henrique da Silva Pires1 1 LaboratoÂrio de Ecologia Comportamental e EvolucËão, Instituto Nacional de Pesquisas da AmazoÃnia, Av. Andre ArauÂjo, Manaus, AM, Brazil, 2 Wildlife Research Center of Kyoto University, Sakyo-ku, Kyoto, Japan a1111111111 a1111111111 * [email protected] a1111111111 a1111111111 a1111111111 Abstract In the absence of constraints, preference for larger mates is expected to evolve, as larger individuals are typical of higher potential fitness. Large females are often more fecund and OPEN ACCESS carry larger eggs (which result in higher number and better quality of offspring), whereas Citation: Borghezan EdA, Pinto KdS, Zuanon J, large males usually have more conspicuous ornaments and are better at defending Pires THdS (2019) Someone like me: Size- resources. However, intrasexual competition can constrain the access to larger partners, assortative pairing and mating in an Amazonian especially when opportunities for mate takeover abound. Here we investigate the relation- fish, sailfin tetra Crenuchus spilurus. PLoS ONE 14 ship between individual's size and mate choice in relation to one's own size and their (9): e0222880. https://doi.org/10.1371/journal. pone.0222880 respective mate's size using the sailfin tetra, a sexually dimorphic Amazonian fish species. We show that ornaments of larger males are exponentially more conspicuous, and larger Editor: John A. B. Claydon, Institute of Marine Research, NORWAY females are more fecund and carry larger eggs. Contrary to expectation, neither males nor females associated for longer with the larger of two offered potential mates. -

Information Sheet on Ramsar Wetlands (RIS) – 2009-2012 Version Available for Download From
Information Sheet on Ramsar Wetlands (RIS) – 2009-2012 version Available for download from http://www.ramsar.org/ris/key_ris_index.htm. Categories approved by Recommendation 4.7 (1990), as amended by Resolution VIII.13 of the 8th Conference of the Contracting Parties (2002) and Resolutions IX.1 Annex B, IX.6, IX.21 and IX. 22 of the 9th Conference of the Contracting Parties (2005). Notes for compilers: 1. The RIS should be completed in accordance with the attached Explanatory Notes and Guidelines for completing the Information Sheet on Ramsar Wetlands. Compilers are strongly advised to read this guidance before filling in the RIS. 2. Further information and guidance in support of Ramsar site designations are provided in the Strategic Framework and guidelines for the future development of the List of Wetlands of International Importance (Ramsar Wise Use Handbook 14, 3rd edition). A 4th edition of the Handbook is in preparation and will be available in 2009. 3. Once completed, the RIS (and accompanying map(s)) should be submitted to the Ramsar Secretariat. Compilers should provide an electronic (MS Word) copy of the RIS and, where possible, digital copies of all maps. 1. Name and address of the compiler of this form: FOR OFFICE USE ONLY. DD MM YY Beatriz de Aquino Ribeiro - Bióloga - Analista Ambiental / [email protected], (95) Designation date Site Reference Number 99136-0940. Antonio Lisboa - Geógrafo - MSc. Biogeografia - Analista Ambiental / [email protected], (95) 99137-1192. Instituto Chico Mendes de Conservação da Biodiversidade - ICMBio Rua Alfredo Cruz, 283, Centro, Boa Vista -RR. CEP: 69.301-140 2. -

A Rapid Biological Assessment of the Upper Palumeu River Watershed (Grensgebergte and Kasikasima) of Southeastern Suriname
Rapid Assessment Program A Rapid Biological Assessment of the Upper Palumeu River Watershed (Grensgebergte and Kasikasima) of Southeastern Suriname Editors: Leeanne E. Alonso and Trond H. Larsen 67 CONSERVATION INTERNATIONAL - SURINAME CONSERVATION INTERNATIONAL GLOBAL WILDLIFE CONSERVATION ANTON DE KOM UNIVERSITY OF SURINAME THE SURINAME FOREST SERVICE (LBB) NATURE CONSERVATION DIVISION (NB) FOUNDATION FOR FOREST MANAGEMENT AND PRODUCTION CONTROL (SBB) SURINAME CONSERVATION FOUNDATION THE HARBERS FAMILY FOUNDATION Rapid Assessment Program A Rapid Biological Assessment of the Upper Palumeu River Watershed RAP (Grensgebergte and Kasikasima) of Southeastern Suriname Bulletin of Biological Assessment 67 Editors: Leeanne E. Alonso and Trond H. Larsen CONSERVATION INTERNATIONAL - SURINAME CONSERVATION INTERNATIONAL GLOBAL WILDLIFE CONSERVATION ANTON DE KOM UNIVERSITY OF SURINAME THE SURINAME FOREST SERVICE (LBB) NATURE CONSERVATION DIVISION (NB) FOUNDATION FOR FOREST MANAGEMENT AND PRODUCTION CONTROL (SBB) SURINAME CONSERVATION FOUNDATION THE HARBERS FAMILY FOUNDATION The RAP Bulletin of Biological Assessment is published by: Conservation International 2011 Crystal Drive, Suite 500 Arlington, VA USA 22202 Tel : +1 703-341-2400 www.conservation.org Cover photos: The RAP team surveyed the Grensgebergte Mountains and Upper Palumeu Watershed, as well as the Middle Palumeu River and Kasikasima Mountains visible here. Freshwater resources originating here are vital for all of Suriname. (T. Larsen) Glass frogs (Hyalinobatrachium cf. taylori) lay their -
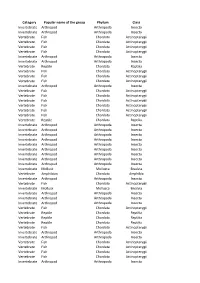
Category Popular Name of the Group Phylum Class Invertebrate
Category Popular name of the group Phylum Class Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Vertebrate Reptile Chordata Reptilia Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Invertebrate Arthropod Arthropoda Insecta Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Reptile Chordata Reptilia Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Mollusk Mollusca Bivalvia Vertebrate Amphibian Chordata Amphibia Invertebrate Arthropod Arthropoda Insecta Vertebrate Fish Chordata Actinopterygii Invertebrate Mollusk Mollusca Bivalvia Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Vertebrate -

Abstracts Part 1
375 Poster Session I, Event Center – The Snowbird Center, Friday 26 July 2019 Maria Sabando1, Yannis Papastamatiou1, Guillaume Rieucau2, Darcy Bradley3, Jennifer Caselle3 1Florida International University, Miami, FL, USA, 2Louisiana Universities Marine Consortium, Chauvin, LA, USA, 3University of California, Santa Barbara, Santa Barbara, CA, USA Reef Shark Behavioral Interactions are Habitat Specific Dominance hierarchies and competitive behaviors have been studied in several species of animals that includes mammals, birds, amphibians, and fish. Competition and distribution model predictions vary based on dominance hierarchies, but most assume differences in dominance are constant across habitats. More recent evidence suggests dominance and competitive advantages may vary based on habitat. We quantified dominance interactions between two species of sharks Carcharhinus amblyrhynchos and Carcharhinus melanopterus, across two different habitats, fore reef and back reef, at a remote Pacific atoll. We used Baited Remote Underwater Video (BRUV) to observe dominance behaviors and quantified the number of aggressive interactions or bites to the BRUVs from either species, both separately and in the presence of one another. Blacktip reef sharks were the most abundant species in either habitat, and there was significant negative correlation between their relative abundance, bites on BRUVs, and the number of grey reef sharks. Although this trend was found in both habitats, the decline in blacktip abundance with grey reef shark presence was far more pronounced in fore reef habitats. We show that the presence of one shark species may limit the feeding opportunities of another, but the extent of this relationship is habitat specific. Future competition models should consider habitat-specific dominance or competitive interactions. -

A New Species of Characidium (Characiformes: Crenuchidae) From
Neotropical Ichthyology, 17(2): e180121, 2019 Journal homepage: www.scielo.br/ni DOI: 10.1590/1982-0224-20180121 Published online: 18 July 2019 (ISSN 1982-0224) Copyright © 2019 Sociedade Brasileira de Ictiologia Printed: 30 June 2019 (ISSN 1679-6225) Original article A new species of Characidium (Characiformes: Crenuchidae) from coastal basins in the Atlantic Rainforest of eastern Brazil, with phylogenetic and phylogeographic insights into the Characidium alipioi species group Evandro Malanski1,2,5, Luisa Maria Sarmento-Soares1,3,6, Ana Cecilia Gomes Silva-Malanski2,5, Maridiesse Morais Lopes3, Leonardo Ferreira da Silva Ingenito1,4 and Paulo Andreas Buckup2 A new species of Characidium from southeastern Brazil is described based on morphological and molecular evidence from specimens collected between the rio Jucuruçu and rio Doce basins. The new species belongs to a group of species within Characidium with an unscaled area in the isthmus and is distinguished from these species, except C. alipioi, C. fasciatum, C. hasemani, and C. kamakan, by the greater distance (greater than 10% SL) and presence of 5-7 scales between the anus and the anal fin, and presence of 14 series of scales around the caudal peduncle. The species is distinguished fromC. alipioi by having 4 series of scales above the lateral line (vs. 5 series) and greater distance between the anus and the anal fin; fromC. fasciatum and C. kamakan, by the smaller body depth at the dorsal-fin origin, at the anal-fin origin, and at the caudal peduncle; from C. hasemani, by the short distances between the tip of the snout and the pelvic fin, the tip of the snout and the anal fin, and the tip of the snout and the tip of anal fin. -

Fossils Provide Better Estimates of Ancestral Body Size Than Do Extant
Acta Zoologica (Stockholm) 90 (Suppl. 1): 357–384 (January 2009) doi: 10.1111/j.1463-6395.2008.00364.x FossilsBlackwell Publishing Ltd provide better estimates of ancestral body size than do extant taxa in fishes James S. Albert,1 Derek M. Johnson1 and Jason H. Knouft2 Abstract 1Department of Biology, University of Albert, J.S., Johnson, D.M. and Knouft, J.H. 2009. Fossils provide better Louisiana at Lafayette, Lafayette, LA estimates of ancestral body size than do extant taxa in fishes. — Acta Zoologica 2 70504-2451, USA; Department of (Stockholm) 90 (Suppl. 1): 357–384 Biology, Saint Louis University, St. Louis, MO, USA The use of fossils in studies of character evolution is an active area of research. Characters from fossils have been viewed as less informative or more subjective Keywords: than comparable information from extant taxa. However, fossils are often the continuous trait evolution, character state only known representatives of many higher taxa, including some of the earliest optimization, morphological diversification, forms, and have been important in determining character polarity and filling vertebrate taphonomy morphological gaps. Here we evaluate the influence of fossils on the interpretation of character evolution by comparing estimates of ancestral body Accepted for publication: 22 July 2008 size in fishes (non-tetrapod craniates) from two large and previously unpublished datasets; a palaeontological dataset representing all principal clades from throughout the Phanerozoic, and a macroecological dataset for all 515 families of living (Recent) fishes. Ancestral size was estimated from phylogenetically based (i.e. parsimony) optimization methods. Ancestral size estimates obtained from analysis of extant fish families are five to eight times larger than estimates using fossil members of the same higher taxa. -

Redalyc.Checklist of the Freshwater Fishes of Colombia
Biota Colombiana ISSN: 0124-5376 [email protected] Instituto de Investigación de Recursos Biológicos "Alexander von Humboldt" Colombia Maldonado-Ocampo, Javier A.; Vari, Richard P.; Saulo Usma, José Checklist of the Freshwater Fishes of Colombia Biota Colombiana, vol. 9, núm. 2, 2008, pp. 143-237 Instituto de Investigación de Recursos Biológicos "Alexander von Humboldt" Bogotá, Colombia Available in: http://www.redalyc.org/articulo.oa?id=49120960001 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Biota Colombiana 9 (2) 143 - 237, 2008 Checklist of the Freshwater Fishes of Colombia Javier A. Maldonado-Ocampo1; Richard P. Vari2; José Saulo Usma3 1 Investigador Asociado, curador encargado colección de peces de agua dulce, Instituto de Investigación de Recursos Biológicos Alexander von Humboldt. Claustro de San Agustín, Villa de Leyva, Boyacá, Colombia. Dirección actual: Universidade Federal do Rio de Janeiro, Museu Nacional, Departamento de Vertebrados, Quinta da Boa Vista, 20940- 040 Rio de Janeiro, RJ, Brasil. [email protected] 2 Division of Fishes, Department of Vertebrate Zoology, MRC--159, National Museum of Natural History, PO Box 37012, Smithsonian Institution, Washington, D.C. 20013—7012. [email protected] 3 Coordinador Programa Ecosistemas de Agua Dulce WWF Colombia. Calle 61 No 3 A 26, Bogotá D.C., Colombia. [email protected] Abstract Data derived from the literature supplemented by examination of specimens in collections show that 1435 species of native fishes live in the freshwaters of Colombia. -

Redalyc.Diet of Two Syntopic Species of Crenuchidae (Ostariophysi
Biota Neotropica ISSN: 1676-0611 [email protected] Instituto Virtual da Biodiversidade Brasil Fernandes, Suzanne; Pereira Leitão, Rafael; Pereira Dary, Eurizângela; Camacho Guerreiro, Ana Isabel; Zuanon, Jansen; Motta Bührnheim, Cristina Diet of two syntopic species of Crenuchidae (Ostariophysi: Characiformes) in an Amazonian rocky stream Biota Neotropica, vol. 17, núm. 1, 2017, pp. 1-6 Instituto Virtual da Biodiversidade Campinas, Brasil Available in: http://www.redalyc.org/articulo.oa?id=199149836015 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Biota Neotropica 17(1): e20160281, 2017 ISSN 1676-0611 (online edition) article Diet of two syntopic species of Crenuchidae (Ostariophysi: Characiformes) in an Amazonian rocky stream Suzanne Fernandes1,2*, Rafael Pereira Leitão3,4, Eurizângela Pereira Dary3, Ana Isabel Camacho Guerreiro1, Jansen Zuanon3 & Cristina Motta Bührnheim2 1Instituto Nacional de Pesquisas da Amazonia, Programa de Pós-Graduação em Biologia de água Doce e Pesca de Interior, Manaus, AM, Brazil 2Universidade do Estado do Amazonas, Escola Normal Superior, Manaus, AM, Brazil 3Instituto Nacional de Pesquisas da Amazônia, Coordenação de Biodiversidade, Manaus, AM, Brazil 4Universidade Federal de Minas Gerais, Instituto de Ciencias Biologicas, Departamento de Biologia Geral, Belo Horizonte, MG, Brazil *Corresponding author: Suzanne Fernandes, e-mail: [email protected] FERNANDES, S., LEITÃO, R.P., DARY, E.P., GUERREIRO, A.I.C., ZUANON, J., BÜHRNHEIM, C., M. Diet of two syntopic species of Crenuchidae (Ostariophysi: Characiformes) in an Amazonian rocky stream. -

Fishes Found Near Pucallpa, Peru Scott Smith
Fishes Found Near Pucallpa, Peru Scott Smith Spring 2017 American Currents 27 FISHES FOUND NEAR PUCALLPA, PERU Scott Smith Morehead City, North Carolina In February 2016, Fritz Rohde, Brian Perkins and I started at the local university aquaculture facility. The facility was planning a trip to collect fishes near the city of Pucallpa, roughly eight air miles southwest of the city center, on the Peru. Pucallpa is a city in the Peruvian Amazon, 300 miles northeast shores of Laguna de Quistococha. To access the fa- northeast of Lima, along the banks of the Ucayali River. cility, a dirt road runs from the main highway to a dirt park- Our plan was to leave in June, use Pucallpa as a home ing lot 200m into the jungle. From there it was another 300m base, and employ a hub and spoke model to sample the of trail and bushwhacking with the help of a local man and surrounding areas. We also decided to make a stopover in his machete. Upon arrival, Fritz stopped to record our GPS Iquitos for a few days, to pick up a Peruvian friend, Jorge. location, while Brian and I got right to fishing. My first swipe Brian, Fritz and I have spent many trips collecting over netted a knifefish (Gymnotidae), which are called “macana” by the last two years, both in the United States, and in Peru, the locals, but was new to us. Further fishing revealed a second but the remoteness of some of our planned Pucallpa areas species of knifefish, a potentially undescribed Rivulus of the promised to make this trip unique. -

New Species Discoveries in the Amazon 2014-15
WORKINGWORKING TOGETHERTOGETHER TO TO SHARE SCIENTIFICSCIENTIFIC DISCOVERIESDISCOVERIES UPDATE AND COMPILATION OF THE LIST UNTOLD TREASURES: NEW SPECIES DISCOVERIES IN THE AMAZON 2014-15 WWF is one of the world’s largest and most experienced independent conservation organisations, WWF Living Amazon Initiative Instituto de Desenvolvimento Sustentável with over five million supporters and a global network active in more than 100 countries. WWF’s Mamirauá (Mamirauá Institute of Leader mission is to stop the degradation of the planet’s natural environment and to build a future Sustainable Development) Sandra Charity in which humans live in harmony with nature, by conserving the world’s biological diversity, General director ensuring that the use of renewable natural resources is sustainable, and promoting the reduction Communication coordinator Helder Lima de Queiroz of pollution and wasteful consumption. Denise Oliveira Administrative director Consultant in communication WWF-Brazil is a Brazilian NGO, part of an international network, and committed to the Joyce de Souza conservation of nature within a Brazilian social and economic context, seeking to strengthen Mariana Gutiérrez the environmental movement and to engage society in nature conservation. In August 2016, the Technical scientific director organization celebrated 20 years of conservation work in the country. WWF Amazon regional coordination João Valsecchi do Amaral Management and development director The Instituto de Desenvolvimento Sustentável Mamirauá (IDSM – Mamirauá Coordinator Isabel Soares de Sousa Institute for Sustainable Development) was established in April 1999. It is a civil society Tarsicio Granizo organization that is supported and supervised by the Ministry of Science, Technology, Innovation, and Communications, and is one of Brazil’s major research centres.