République Démocratique Du Congo
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Democratic Republic of Congo Constitution
THE CONSTITUTION OF THE DEMOCRATIC REPUBLIC OF THE CONGO, 2005 [1] Table of Contents PREAMBLE TITLE I GENERAL PROVISIONS Chapter 1 The State and Sovereignty Chapter 2 Nationality TITLE II HUMAN RIGHTS, FUNDAMENTAL LIBERTIES AND THE DUTIES OF THE CITIZEN AND THE STATE Chapter 1 Civil and Political Rights Chapter 2 Economic, Social and Cultural Rights Chapter 3 Collective Rights Chapter 4 The Duties of the Citizen TITLE III THE ORGANIZATION AND THE EXERCISE OF POWER Chapter 1 The Institutions of the Republic TITLE IV THE PROVINCES Chapter 1 The Provincial Institutions Chapter 2 The Distribution of Competences Between the Central Authority and the Provinces Chapter 3 Customary Authority TITLE V THE ECONOMIC AND SOCIAL COUNCIL TITLE VI DEMOCRACY-SUPPORTING INSTITUTIONS Chapter 1 The Independent National Electoral Commission Chapter 2 The High Council for Audiovisual Media and Communication TITLE VII INTERNATIONAL TREATIES AND AGREEMENTS TITLE VIII THE REVISION OF THE CONSTITUTION TITLE IX TRANSITORY AND FINAL PROVISIONS PREAMBLE We, the Congolese People, United by destiny and history around the noble ideas of liberty, fraternity, solidarity, justice, peace and work; Driven by our common will to build in the heart of Africa a State under the rule of law and a powerful and prosperous Nation based on a real political, economic, social and cultural democracy; Considering that injustice and its corollaries, impunity, nepotism, regionalism, tribalism, clan rule and patronage are, due to their manifold vices, at the origin of the general decline -

ACTIVE USG PROGRAMS for the DEMOCRATIC REPUBLIC of the CONGO RESPONSE Last Updated 07/27/20
ACTIVE USG PROGRAMS FOR THE DEMOCRATIC REPUBLIC OF THE CONGO RESPONSE Last Updated 07/27/20 BAS-UELE HAUT-UELE ITURI S O U T H S U D A N COUNTRYWIDE NORTH KIVU OCHA IMA World Health Samaritan’s Purse AIRD Internews CARE C.A.R. Samaritan’s Purse Samaritan’s Purse IMA World Health IOM UNHAS CAMEROON DCA ACTED WFP INSO Medair FHI 360 UNICEF Samaritan’s Purse Mercy Corps IMA World Health NRC NORD-UBANGI IMC UNICEF Gbadolite Oxfam ACTED INSO NORD-UBANGI Samaritan’s WFP WFP Gemena BAS-UELE Internews HAUT-UELE Purse ICRC Buta SCF IOM SUD-UBANGI SUD-UBANGI UNHAS MONGALA Isiro Tearfund IRC WFP Lisala ACF Medair UNHCR MONGALA ITURI U Bunia Mercy Corps Mercy Corps IMA World Health G A EQUATEUR Samaritan’s NRC EQUATEUR Kisangani N Purse WFP D WFPaa Oxfam Boende A REPUBLIC OF Mbandaka TSHOPO Samaritan’s ATLANTIC NORTH GABON THE CONGO TSHUAPA Purse TSHOPO KIVU Lake OCEAN Tearfund IMA World Health Goma Victoria Inongo WHH Samaritan’s Purse RWANDA Mercy Corps BURUNDI Samaritan’s Purse MAI-NDOMBE Kindu Bukavu Samaritan’s Purse PROGRAM KEY KINSHASA SOUTH MANIEMA SANKURU MANIEMA KIVU WFP USAID/BHA Non-Food Assistance* WFP ACTED USAID/BHA Food Assistance** SA ! A IMA World Health TA N Z A N I A Kinshasa SH State/PRM KIN KASAÏ Lusambo KWILU Oxfam Kenge TANGANYIKA Agriculture and Food Security KONGO CENTRAL Kananga ACTED CRS Cash Transfers For Food Matadi LOMAMI Kalemie KASAÏ- Kabinda WFP Concern Economic Recovery and Market Tshikapa ORIENTAL Systems KWANGO Mbuji T IMA World Health KWANGO Mayi TANGANYIKA a KASAÏ- n Food Vouchers g WFP a n IMC CENTRAL y i k -

UNJHRO) MONUSCO – OHCHR March 2021 REPORTED HUMAN RIGHTS VIOLATIONS in DEMOCRATIC REPUBLIC of the CONGO (DRC)
Protection of civilians: Human rights violations documented in provinces affected by conflict United Nations Joint Human Rights Office in the DRC (UNJHRO) MONUSCO – OHCHR March 2021 REPORTED HUMAN RIGHTS VIOLATIONS IN DEMOCRATIC REPUBLIC OF THE CONGO (DRC) Figure 1. Percentage of violations per territory Figure 2. Number of violations per province in DRC SOUTH CENTRAL AFRICAN REPUBLIC SUDAN North Kivu Tanganyika Bas-Uele Haut-Uele Masisi 79% 21 Kalemie 36% 65 North-Ubangi Beni 64 36 Manono0 100 2 UGANDA CAMEROON South-Ubangi Rutshuru 69 31 Moba0 100 Ituri Mongala Lubero 29 71 77 Nyiragongo 86 14 Maniema Tshopo Walikale 90 10 Kabambare 63% 395 CONGO Equateur North Butembo0 100 Kasongo0 100 Kivu Kibombo0 100 GABON Tshuapa 359 South Kivu RWANDA Kasai Shabunda 82% 18 Mai-Ndombe Kamonia (Kas.)0 100% Kinshasa Uvira 33 67 5 BURUNDI Llebo (Kas.)0 100 Sankuru 15 63 Fizi 33 67 Kasai South Tshikapa (Kas.)0 100 Maniema Kivu Kabare 100 0 Luebo (Kas.)0 100 Kwilu 23 TANZANIA Walungu 29 71 Kananga (Kas. C)0 100 Lomami Bukavu0 100 22 4 Demba (Kas. C)0 100 Kongo 46 Mwenga 67 33 Central Luiza (Kas. C)0 100 Kwango Tanganyika Kalehe0 100 Kasai Dimbelenge (Kas. C)0 100 Central Haut-Lomami Ituri Miabi (Kas. O)0 100 Kasai 0 100 ANGOLA Oriental Irumu 88% 12 Mbuji-Mayi (Kas. O) Haut- Djugu 64 36 Lualaba Bas-Uele Katanga Mambasa 30 70 Buta0 100% Mahagi 100 0 % by armed groups % by State agents The boundaries and names shown and designations ZAMBIA used on this map do not imply official endorsement or acceptance by the United Nations. -
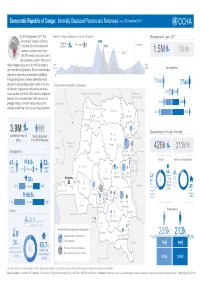
FINAL-Fact-Sheet-Infographic-19-Oct-2017 English Version.Ai
Democratic Republic of Congo: Internally Displaced Persons and Returnees (as of 30 September 2017) As of 30 September 2017, the Monthly trend of displacement in last 18 months Displacement in year 2017 Democratic Republic of Congo 432k Displaced Returnees 3rd Quarter recorded 3.9 million displaced persons 388k persons, including more than 2017 1.5M 1M 400,000 newly displaced ones in the prior three months. With over 1 million displaced persons, North Kivu remains April Sept. Demographics the most affected province. Recent months have 2016 2017 also seen large return movements, notably in 0 Tanganyika province, where authorities have 714k decided to vacate displacement sites in the city Displacement distribution by province 774k of Kalemie. In provinces affected by the Kasai 27k 29k crisis, as well, some 631,000 internally displaced CENTRAL AFRICAN REPUBLIC REPUBLIC OF persons have returned home, often to burnt or SOUTH SUDAN 263k 285k pillaged villages. Overall, armed attacks and CAMEROON clashes remain the main cause of displacement. North-Ubangi Bas-Uele 2 424k 460k Haut-Uele South-Ubangi Mongala 3.9M 45 Displacement in the last 3 months current number of Ituri 325 forcibly displaced DrDraff UGANDA 42 IDPs in the affected areas Equateur 24 REPUBLIC OF Tshopo CONGO GABON 428k 213k Demographics Tshuapa 1 024 267 North Kivu RWANDA Causes Nature of accomodation 48% 4.5% 52% Maï-Ndombe men >59 years women 71k 77k BURUNDI (1.9M) (2M) 21 % 254 45 545 104 55 Maniema South Kivu Clashes Kinshasa Sankuru and % % % 13 35 45 armed sites 30 Inter- -

Democratic Republic of Congo
DEMOCRATIC REPUBLIC OF CONGO 350 Fifth Ave 34 th Floor New York, N.Y. 10118-3299 http://www.hrw.org (212) 290-4700 Vol. 15, No. 11 (A) - July 2003 I hid in the mountains and went back down to Songolo at about 3:00 p.m. I saw many people killed and even saw traces of blood where people had been dragged. I counted 82 bodies most of whom had been killed by bullets. We did a survey and found that 787 people were missing – we presumed they were all dead though we don’t know. Some of the bodies were in the road, others in the forest. Three people were even killed by mines. Those who attacked knew the town and posted themselves on the footpaths to kill people as they were fleeing. -- Testimony to Human Rights Watch ITURI: “COVERED IN BLOOD” Ethnically Targeted Violence In Northeastern DR Congo 1630 Connecticut Ave, N.W., Suite 500 2nd Floor, 2-12 Pentonville Road 15 Rue Van Campenhout Washington, DC 20009 London N1 9HF, UK 1000 Brussels, Belgium TEL (202) 612-4321 TEL: (44 20) 7713 1995 TEL (32 2) 732-2009 FAX (202) 612-4333 FAX: (44 20) 7713 1800 FAX (32 2) 732-0471 E-mail: [email protected] E-mail: [email protected] E-mail: [email protected] “You cannot escape from the horror” This story of fifteen-year-old Elise is one of many in Ituri. She fled one attack after another and witnessed appalling atrocities. Walking for more than 300 miles in her search for safety, Elise survived to tell her tale; many others have not. -

Province De L'equateur Profil Resume Pauvrete Et
Programme des nations unies pour le développement Unité de lutte contre la pauvreté RDC PROVINCE DE L’EQUATEUR PROFIL RESUME PAUVRETE ET CONDITIONS DE VIE DES MENAGES Mars 2009 - 1 – Province de L’Equateur Sommaire PROVINCE DE L’EQUATEUR Province Equateur Avant-propos..............................................................3 Superficie 403.292km² 1 – Présentation générale de l’Equateur....................4 Population 2005 5,8 millions 2 – La pauvreté dans l’Equateur ................................6 Densité 14 hab/km² Nb de communes 7 3 – L’éducation.........................................................10 Nb de territoires 8 4 – Le développement socio-économique des Routes d’intérêt national 2.939 km femmes.....................................................................11 Routes d’intérêt provincial 2.716 km 5 – La malnutrition et la mortalité infantile ...............12 Routes secondaires 3.158 km 6 – La santé maternelle............................................13 Réseau ferroviaire 187 km 7 – Le sida et le paludisme ......................................14 Gestion de la province Gouvernement Provincial 8 – L’habitat, l’eau et l’assainissement ....................15 Nb de ministres provinciaux 10 9 – Le développement communautaire et l’appui des Nb de députés provinciaux 108 Partenaires Techniques et Financiers (PTF) ...........16 - 2 – Province de L’Equateur Avant-propos Le présent rapport présente une analyse succincte des conditions de vie des ménages de la province de l’Equateur. L’analyse se base essentiellement sur les récentes enquêtes statistiques menées en RDC. Il fait partie d’une série de documents sur les conditions de vie de la population des 11 provinces de la RDC. Cette série de rapports constitue une analyse réalisée en toute indépendance par des experts statisticiens-économistes, afin de fournir une vision objective de la réalité de chaque province en se basant sur les principaux indicateurs de pauvreté et conditions de vie de la population, spécialement ceux se rapportant aux OMD et à la stratégie de réduction de la pauvreté. -
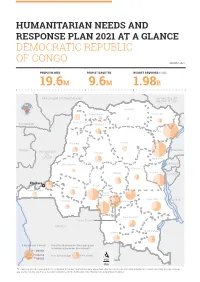
Drc Hno-Hrp 2021 at a Glance.Pdf
HUMANITARIAN NEEDS AND RESPONSE PLAN 2021 AT A GLANCE DEMOCRATIC REPUBLIC OF CONGO JANUARY 2021 PEOPLE IN NEED PEOPLE TARGETED BUDGET REQUIRED (USD) 19.6M 9.6M 1.98B RÉPUBLIQUE CENTRAFRICAINE RÉPUBLIQUE DU SOUDAN DU SUD Bas-Uele Nord-Ubangi Haut-Uele Sud-Ubangi CAMEROUN Mongala Ituri Equateur Tshopo GABON OUGANDA RÉPUBLIQUE Tshuapa DU CONGO Nord-Kivu Maï-Ndombe RWANDA Maniema Sud-Kivu Sankuru BURUNDI insasa Kasaï Kwilu Kongo-Central Kasaï- Central Tanganyika TANZANIE Kwango Lomami Haut-Lomami Kasaï- Oriental ANGOLA Haut-Katanga Lualaba # de pers.dans le besoin Proportion de personnes ciblées par rapport au nombre de personnes dans le besoin 1 000 000 500 000 Pers. dans le besoin Pers. ciblées O 100 000 100 Km The names used in the report and the presentation of the various media do not imply any opinion whatsover on the part of the United Nations Secretariat concerning the legal status of any country, territory, city or area, or of their authorities, nor the delimitation of its boundaries or geographical boundaries. ZAMBIE HUMANITARIAN NEEDS AND RESPONSE PLAN 2020 AT A GLANCE Severity of needs INTERSECTORAL SEVERITY OF NEEDS (2021) MINOR MODERATE STRICT CRITICAL CATASTROPHIC 11% 17% 44% 24% 4% CENTRAL AFRICAN REPUBLIC REPUBLIC OF SOUTH SUDAN CAMEROON Nord-Ubangi Sud-Ubangi Haut-Uele Bas-Uele Mongala Ituri Equateur Tshopo REPUBLIC OF Tshuapa OUGANDA CONGO GABON Nord-Kivu RWANDA Maï-Ndombe Maniema Sud-Kivu BURUNDI Kinshasa Sankuru !^ Kwilu Kasaï Kongo-Central Lomami Kasaï- TANZANIA Central Tanganyika Kwango Kasaï- Oriental Haut-Lomami Haut-Katanga Lualaba ANGOLA Severity of needs O Catastrophic Critical 100 Strict Km ZAMBIA Moderate Minor None The designations employed in the report and the presentation of the various materials do not imply any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of countries, territories, cities or areas, or of their authorities, nor of the delimitation of its frontiers or geographical limits. -

Democratic Republic of the Congo Insight and Key Trends by the World Food Programme (WFP) | 1 August 2021
HungerMapLIVE: Democratic Republic of the Congo insight and key trends By the World Food Programme (WFP) | 1 August 2021 FOOD INSECURITY AT A GLANCE 18.5M M 26.2M Chronic hunger 105.9 Acute hunger Population (undernourishment) (WFP D.R. Congo, 2021) IPC Phase 3+ (SOFI Report, 2021)1 (IPC, Aug 2021 - Dec 2021)2 The HungerMapLIVE tracks core indicators of acute hunger in near real-time. Acute hunger is measured by key indicators such as household food consumption, livelihood behaviors, child nutritional status, mortality, access to clean drinking water and other contextual factors. The HungerMapLIVE primarily tracks trends on household food consumption, consumption-based coping and livelihood changes to track multiple aspects of food insecurity. As these are outcome level 1 indicators in the Integrated Food Security Phase Classication (IPC) Framework, they can provide early indications of potential shifts in acute food insecurity. Insucient food consumption (HungerMapLIVE data)3 36.9M → 42.3M As of 3 May 2021 As of 1 August 2021 Crisis or above crisis level food-based coping strategies (HungerMapLIVE data)3 52.6M → 49.1M As of 3 May 2021 As of 1 August 2021 Methodology Note: The HungerMapLIVE includes data from two sources: (1) WFP’s continuous, near real-time monitoring systems, which remotely collect thousands of data daily through live calls conducted by call centres around the world; and (2) machine learning-based predictive models. Therefore, to note this dierentiation, this report indicates whether a region’s data is based on WFP’s near real-time monitoring systems (marked ‘ACTUAL’) or predictive models (marked ‘PREDICTED’). -

Use of Iris Scanning for Biometric Recognition of Healthy Adults Participating in an Ebola Vaccine Trial in the Democratic Republic of the Congo: Mixed Methods Study
JOURNAL OF MEDICAL INTERNET RESEARCH Zola Matuvanga et al Original Paper Use of Iris Scanning for Biometric Recognition of Healthy Adults Participating in an Ebola Vaccine Trial in the Democratic Republic of the Congo: Mixed Methods Study Trésor Zola Matuvanga1,2,3, MD; Ginger Johnson4, PhD; Ynke Larivière2,3, MPH; Emmanuel Esanga Longomo5, MD; Junior Matangila1, PhD; Vivi Maketa1, PhD; Bruno Lapika6, PhD; Patrick Mitashi1, PhD; Paula Mc Kenna7, MSc; Jessie De Bie2,3, PhD; Jean-Pierre Van Geertruyden2, PhD; Pierre Van Damme3, PhD; Hypolite Muhindo Mavoko1, PhD 1Department of Tropical Medicine, University of Kinshasa, Kinshasa, the Democratic Republic of the Congo 2Global Health Institute, University of Antwerp, Antwerp, Belgium 3Centre for the Evaluation of Vaccination, University of Antwerp, Antwerp, Belgium 4Department of Public Health, Institute of Tropical Medicine, Antwerp, Belgium 5Division Provinciale de la Santé de la Province de la Tshuapa, Boende, the Democratic Republic of the Congo 6Department of Anthropology, University of Kinshasa, Kinshasa, the Democratic Republic of the Congo 7Janssen Pharmaceutica NV, Beerse, Belgium Corresponding Author: Trésor Zola Matuvanga, MD Department of Tropical Medicine University of Kinshasa Avenue Université numéro 1, Commune de Lemba Kinshasa the Democratic Republic of the Congo Phone: 243 810046306 Email: [email protected] Abstract Background: A partnership between the University of Antwerp and the University of Kinshasa implemented the EBOVAC3 clinical trial with an Ebola vaccine regimen administered to health care provider participants in Tshuapa Province, Democratic Republic of the Congo. This randomized controlled trial was part of an Ebola outbreak preparedness initiative financed through Innovative Medicines Initiative-European Union. -

Democratic Republic of the Congo [email protected] Livestock Practices, Management of Input Shops and Community Warehouses
Democratic Republic of the Congo Response overview | May 2021 • The Democratic Republic of Congo has the highest estimated number of people in acute food insecurity worldwide. The key drivers of food insecurity in the country are protracted conflict, mainly in the eastern provinces triggering population displacements and the disruption of livelihoods, the effects of coronavirus disease 2019 (COVID-19) and related containment measures, the economic decline linked to the currency depreciation and drop in GDP growth, and natural hazards (floods, animal diseases, etc.). • The influx of about 92 000 refugees from the Central African Republic in early 2021 are increasing pressure on an already difficult humanitarian situation in North and South Ubangui. • The Government declared a state of emergency in the North-Kivu and Ituri provinces following extreme violence against the civilian population perpetrated by non-state actors. The provincial authorities and governments will be replaced by officers of the army and of the national police for an initial period of 30 days. © FAO • In line with COVID-19 mitigation measures and recovery efforts, the Food and Agriculture Organization of the United Nations (FAO) is supporting smallholders in Kinshasa, Kasai, Kasai-Central and Ituri 27.3 million people thanks to generous contributions from the Governments of Belgium and in high acute food insecurity Canada, and the Central Emergency Response Fund (CERF). (Phase 3+) (Integrated Food Security Phase Classification [IPC], February–July 2021) It is crucial -
Democratic Republic of the Congo
Democratic Republic of the Congo Humanitarian Situation Report No. 01 © UNICEF/Naftalin 2019 Reporting Period: January 2020 Highlights Situation in Numbers • UNICEF Democratic Republic of the Congo (DRC) launched it’s 9,100,000 2020 Humanitarian Action for Children (HAC) with a funding appeal children in need of of $262 million to support UNICEF’s nutrition, health, WASH, child humanitarian assistance protection, education, Rapid Response, and communication for (OCHA, HNO 2020) development response • In 2020, the number of people in need of humanitarian assistance 15,600,000 increased to 15.6 million, compared to 12.8 million people in 2019 people in need (OCHA, HNO 2020) • The resurgence of violence in Ituri province has forced more than 200,000 new arrivals in spontaneous Internally Displaced Persons (IDPs) sites surrounding Djugu, Mahagi, and Irumu territory. It is 1,100,000 estimated that over 750,000 persons have been displaced since the Internally displaced people beginning of the crisis (HNO 2019) 2,651 cases of cholera reported since January (Ministry of Health) UNICEF’s Response and Funding Status UNICEF Appeal 2020 1% US$ 262 million 11% 0% Funding Status (in US$) Funds 5% received Carry- 2% $11.4 M forward, $25.9M 16% 15% 26% Funding gap, 5% $216.3 M 3% 0% 20% 40% 60% 80% 100% 1 Funding Overview and Partnerships UNICEF appeals for US$ 262M to sustain the provision of humanitarian services for women and children in the Democratic Republic of the Congo (DRC). UNICEF expresses its sincere gratitude to all public and private donors for the contributions received to date. -

The Rise and Decline of the Congolese State
Working Paper No. 21 THE RISE AND DECLINE OF THE CONGOLESE STATE AN ANALYTICAL NARRATIVE ON STATE-MAKING Gabi Hesselbein Crisis States Research Centre November 2007 Copyright © G. Hesselbein 2007 Although every effort is made to ensure the accuracy and reliability of material published in this Working Paper, the Crisis States Research Centre and LSE accept no responsibility for the veracity of claims or accuracy of information provided by contributors. All rights reserved. No part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means without the prior permission in writing of the publisher nor be issued to the public or circulated in any form other than that in which it is published. Requests for permission to reproduce this Working Paper, of any part thereof, should be sent to: The Editor, Crisis States Research Centre, DESTIN, LSE, Houghton Street, London WC2A 2AE. Crisis States Working Papers Series No.2 ISSN 1749-1797 (print) ISSN 1749-1800 (online) 1 Crisis States Research Centre The Rise and Decline of the Congolese State1 An analytical narrative on state-making Gabi Hesselbein2 Crisis States Research Centre Abstract: This analytical narrative investigates the varying nature and strength of the state in Congo/Zaire from colonial times through the tumultuous years after independence, the ups and downs of the Mobutu years, the two wars at the turn of the century, the interim government and the beginning of the fourth republic in 2006. After a discussion of the prevalent theories of state failure, this text discusses state resilience and fragility within the framework of late industrialisation and the difficulties in transforming a pre- capitalist society into a capitalist one.