Thesis Effect of Genotype, Storage and Processing On
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Report of a Working Group on Potato: First Meeting, 23-25 March 2000
European Cooperative Programme for Crop Genetic Report Resources Networks ECP GR of a Working Group on Potato First Meeting 23–25 March 2000, Wageningen, The Netherlands R. Hoekstra, L. Maggioni and E. Lipman, compilers <www.futureharvest.org> IPGRI is a Future Harvest Centre supported by the Consultative Group on International Agricultural Research (CGIAR) Report ECP GR of a working group on Potato First Meeting 23–25 March 2000, Wageningen, The Netherlands R. Hoekstra, L. Maggioni and E. Lipman, compilers The International Plant Genetic Resources Institute (IPGRI) is an autonomous international scientific organization, supported by the Consultative Group on International Agricultural Research (CGIAR). IPGRI's mandate is to advance the conservation and use of genetic diversity for the well-being of present and future generations. IPGRI's headquarters is based in Maccarese, near Rome, Italy, with offices in another 19 countries worldwide. The Institute operates through three programmes: (1) the Plant Genetic Resources Programme, (2) the CGIAR Genetic Resources Support Programme and (3) the International Network for the Improvement of Banana and Plantain (INIBAP). The international status of IPGRI is conferred under an Establishment Agreement which, by January 2001, had been signed and ratified by the Governments of Algeria, Australia, Belgium, Benin, Bolivia, Brazil, Burkina Faso, Cameroon, Chile, China, Congo, Costa Rica, Côte d’Ivoire, Cyprus, Czech Republic, Denmark, Ecuador, Egypt, Greece, Guinea, Hungary, India, Indonesia, Iran, Israel, Italy, Jordan, Kenya, Malaysia, Mauritania, Morocco, Norway, Pakistan, Panama, Peru, Poland, Portugal, Romania, Russia, Senegal, Slovakia, Sudan, Switzerland, Syria, Tunisia, Turkey, Uganda and Ukraine. In 2000 financial support for the Research Agenda of IPGRI was provided by the Governments of Armenia, Australia, Austria, Belgium, Brazil, Bulgaria, Canada, China, Croatia, Cyprus, Czech Republic, Denmark, Estonia, F.R. -

Potatoes in the Home Garden
for the Gardener Growing Potatoes in the Home Garden f you could cultivate a vegetable crop that could be grown in almost every climate (except hot tropical zones) from sea level to 15,000 feet, could be eaten for breakfast, lunch, dinner, and snacks, prepared in a myriad of ways, be easily kept Iwithout processing or refrigeration for up to 6-8 months, produced high yields (2-5 pounds per square foot) and was extremely nutritious (high in protein, vitamin C, niacin, B vitamins, iron and energy) but low in calories (sans butter and sour cream), you would wouldn’t you? If you did you would be in the minority of home gardeners. Most gardeners eschew the illustrious “spud” (Solanum tuberosum), thinking it doesn’t warrant space in the small garden and that home grown potatoes don’t taste much better than their store-bought counterparts. Not true! Wrong on both counts. Solanum tuberosum (the Andean potato) originated in the highlands of the Andes mountain ranges of South America (Peru, Columbia, Ecuador, Bolivia) at elevations up to 15,000 feet. Potatoes have been in cultivation for more than 2000 years and there are more than 2,000-3,000 Beveridge Melisa varieties extant today. It is an herbaceous perennial in its native habitat, but treated as a tender annual in the temperate zones and damaged by frost at 28-30°F. The plant’s only edible portions are the tubers produced underground, apically (at the tip) on stolons (horizontal underground stems; see drawing at right). While potatoes produce viable seed, the genetic makeup of sexually- produced plants is so diverse and variable (heterozygous) that production from this seed is negligible. -

Potato - Wikipedia, the Free Encyclopedia
Potato - Wikipedia, the free encyclopedia Log in / create account Article Talk Read View source View history Our updated Terms of Use will become effective on May 25, 2012. Find out more. Main page Potato Contents From Wikipedia, the free encyclopedia Featured content Current events "Irish potato" redirects here. For the confectionery, see Irish potato candy. Random article For other uses, see Potato (disambiguation). Donate to Wikipedia The potato is a starchy, tuberous crop from the perennial Solanum tuberosum Interaction of the Solanaceae family (also known as the nightshades). The word potato may Potato Help refer to the plant itself as well as the edible tuber. In the region of the Andes, About Wikipedia there are some other closely related cultivated potato species. Potatoes were Community portal first introduced outside the Andes region four centuries ago, and have become Recent changes an integral part of much of the world's cuisine. It is the world's fourth-largest Contact Wikipedia food crop, following rice, wheat and maize.[1] Long-term storage of potatoes Toolbox requires specialised care in cold warehouses.[2] Print/export Wild potato species occur throughout the Americas, from the United States to [3] Uruguay. The potato was originally believed to have been domesticated Potato cultivars appear in a huge variety of [4] Languages independently in multiple locations, but later genetic testing of the wide variety colors, shapes, and sizes Afrikaans of cultivars and wild species proved a single origin for potatoes in the area -
Seed Potato Directory 2017
The farm operation grows 93 acres of field generations one and two seed, operates 4 greenhouses producing conventional and NFT minitubers. Our stewardship of this seed continues through WISCONSIN the certification Our of stewardship these seed oflots this on seed Wisconsin continues seed through grower t farms, there is no other program like it. CERTIFIED The program maintains variety trueness to type; selecting and testing clones, rogueing of weak, genetic variants, and diseased plants to continue to develop and maintain germplasm of your SEED POTATOES favorite varieties at our laboratory. 103 Years of Seed Growing Tradition A Century Long Tradition Pioneers In Seed Potato Certification Administered since inception by the College of Agricultural and Life Sciences, University of Wisconsin – Madison, the program Much of the early research work on potato diseases and how retains a full-time staff of experienced professionals to ensure they spread was done Scientists in Germany found and that, Holland through around careful the monitoring turn thoroughness and impartiality in inspection and certification of the century. Scientists found that, through careful monitoring procedures. o of the crop and removal of unhealthy plants, Similar they could research maintain soon was a vigorous, healthy stock indefinitely. Similar research soon was Through providing information, exercising technical skill, doing b being conducted in the United States. research directed at solving problems, and conducting outreach activities, the University meets the growers at the field level. USDA plant pathologist W.A. Orton had studied potato This special relationship to the academic community brings new certification in Germany and upon his return, began to work with T information on pathogens, best practices, and introduces high potato growers and Universities to introduce those concepts quality basic seed into the marketplace. -

Common Scab Susceptibility of 24 Most Popular Potato Cultivars in USA, Utilizing a Greenhouse Assay with Three Different Pathoge
Common scab susceptibility of 24 most popular potato cultivars in USA, utilizing a greenhouse assay with three different pathogenic Streptomyces strains (species) Increasing disease score 0 100 200 300 400 500 600 0 100 200 300 400 500 600 0 100 200 300 400 500 600 Norland No data R Norkotah (ND) R Norkotah (ID) Shepody R Norkotah (ND) Ranger Russet No data R Norkotah (ID) R Norkotah 296 R Norkotah ID Norkotah 3 Red La Soda Shepody Yukon Gold Norkotah 8 Shepody Premier Russet Alturas Norkotah 8 Pike Premier Russet Dk Red Norland Norland Yukon Gold Norkotah 3 Russet Burbank Red La Soda Atlantic R Norkotah 296 Russet Burbank Ranger Russet Gold Rush Dk Red Norland Red La Soda Alturas R Norkotah 296 Megachip Snowden Superior Atlantic Superior Yukon Gold Snowden Russet Burbank Megachip Silverton russet Megachip Rio Grande Yukon Gold ME Dakota Pearl Atlantic Canela russet Dakota Pearl Premier Russet Yukon Gold (ID) Norkotah 3 Norland Dakota Pearl Snowden Silverton russet Superior Canela russet Dk Red Norland Pike R Norkotah ND Yukon Gold (WI) S. scabies Blazer Russet S. stelliscabiei Gold Rush S. species IdX Pike Rio Grande Alturas ME01-11h NY02-1c ID01-12c Gold Rush Yukon Gold 5.1e8 CFU/pot Norkotah 8 1.2e9 CFU/pot Blazer Russet 1e9 CFU/pot Ranger Russet Silverton russet Rio Grande Canela russet Blazer Russet Cultivars are listed along the left side of graphs, ranked by disease severity, with most susceptible at the top and most resistant at the bottom. Disease score is a combination of type of lesion (surface, pits or raised lesions) and amount of surface area affected. -

Potato Glossary
A Potato Glossary A Potato Glossary by Richard E. Tucker Last revised 15 Sep 2016 Copyright © 2016 by Richard E. Tucker Introduction This glossary has been prepared as a companion to A Potato Chronology. In that work, a self-imposed requirement to limit each entry to a single line forced the use of technical phrases, scientific words, jargon and terminology that may be unfamiliar to many, even to those in the potato business. It is hoped that this glossary will aid those using that chronology, and it is hoped that it may become a useful reference for anyone interested in learning more about potatoes, farming and gardening. There was a time, a century or more ago, when nearly everyone was familiar with farming life, the raising of potatoes in particular and the lingo of farming in general. They were farmers themselves, they had relatives who farmed, they knew someone who was a farmer, or they worked on a nearby farm during their youth. Then, nearly everyone grew potatoes in their gardens and sold the extra. But that was a long ago time. Now the general population is now separated from the farm by several generations. Only about 2 % of the US population lives on a farm and only a tiny few more even know anyone who lives on a farm. Words and phrases used by farmers in general and potato growers in particular are now unfamiliar to most Americans. Additionally, farming has become an increasingly complex and technical endeavor. Research on the cutting edge of science is leading to new production techniques, new handling practices, new varieties, new understanding of plant physiology, soil and pest ecology, and other advances too numerous to mention. -

Potatoes in the Garden Dan Drost Vegetable Specialist Summary Potatoes Prefer a Sunny Location, Long Growing Season, and Fertile, Well-Drained Soil for Best Yields
Revised April 2020 Potatoes in the Garden Dan Drost Vegetable Specialist Summary Potatoes prefer a sunny location, long growing season, and fertile, well-drained soil for best yields. Plant potato seed pieces directly in the garden 14-21 days before the last frost date. For earlier maturity, plant potatoes through a black plastic mulch. Side dress with additional nitrogen fertilizer to help grow a large plant. Irrigation should be deep and frequent. Organic mulches help conserve water, reduce weeding, and keep the soil cool during tuber growth. Control insect and diseases throughout the year. Harvest potatoes as soon as tubers begin forming (new potatoes) or as they mature. Dig storage potatoes after the vines have died, cure them for 2-3 weeks, and then store the tubers in the dark at 40-45ºF. Recommended Varieties Potatoes are categorized by maturity class (early, mid-season or late), use (baking, frying, boiling), or tuber skin characteristics (russet, smooth, or colored). When selecting varieties, consider your growing environment, primary use, and how much space you have available to grow the plants. Most varieties grow well in Utah but all are not available. Most garden centers and nurseries carry varieties that produce high quality, productive seed tubers adapted to local conditions. Skin Type Suggested Varieties Russet Butte, Gem Russet, Ranger Russet, Russet Burbank Smooth Chipeta, Katahdin, Kennebec, Yukon Gold All Blue, Caribe (blue), Cranberry Red, Red Norland, Red Pontiac, Rose Finn, Colored Viking, How to Grow Soil: Potatoes prefer organic, rich, well-drained, sandy soil for best growth. Most soils in Utah will grow potatoes provided they are well drained and fertile. -
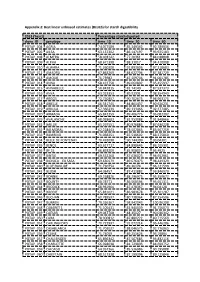
Percentage Starch Digested Clone ID
Appendix 2: Best linear unbiased estimates (BLUES) for starch digestibility 2013 (Year1) Percentage starch digested clone_ID genotype time_10 time_20 time_60 POTAP_004 AGRIA 74.077589 85.349342 92.789936 POTAP_005 AILSA 63.137302 86.147197 93.925183 POTAP_008 ALMERA 58.028142 77.20361 84.038808 POTAP_009 ALPHA 68.671398 88.31017 94.410008 POTAP_010 ALWARA 71.260208 92.893806 97.218683 POTAP_011 AMADRA 67.863269 84.622794 87.381729 POTAP_013 AMOUR 71.73982 87.109706 91.001785 POTAP_014 ANNA 68.422758 89.660892 95.455937 POTAP_015 ANNABELLE 58.843815 82.74149 87.537373 POTAP_016 ANYA 63.923353 89.92991 98.354949 POTAP_017 ARGOS 72.750357 90.702675 98.963175 POTAP_018 ARIELLE 66.341745 84.861437 90.926231 POTAP_019 ARKULA 67.366239 89.333466 92.377485 POTAP_020 ARMADA 65.842359 89.406775 95.082302 POTAP_029 AVALANCHE 68.038683 81.953088 91.848666 POTAP_031 BAILLIE 65.297551 80.976885 87.636228 POTAP_032 BALMORAL 62.228443 78.502863 83.567259 POTAP_033 BAMBINO 71.038251 87.518602 88.375869 POTAP_034 BELLE DE FONTENAY 70.492098 90.744399 93.04301 POTAP_035 BENOL 63.617717 84.898645 86.105301 POTAP_036 BF 15 66.893209 85.908057 93.4925 POTAP_037 BINTJE 69.532268 87.636023 94.952961 POTAP_038 BIONICA EX SASA 69.630179 89.676473 98.449329 POTAP_040 BLUE DANUBE 71.730754 89.36174 96.121599 POTAP_041 BLUSH 64.03457 87.411882 93.846979 POTAP_042 BONNIE 69.258826 88.296975 95.359672 POTAP_043 BOUNTY 68.78771 86.568594 95.651311 POTAP_044 BRODICK 68.961964 87.478367 94.04128 POTAP_045 BRODIE 65.831021 86.983678 95.301287 POTAP_046 BUCHAN 60.789597 -

Relative Salinity Tolerance of Potato Cultivars Assessed by <Emphasis Type="Italic">In Vitro </Emphasis> S
Amer J of Potato Res (1998) 75:207-210 207 Relative Salinity Tolerance of Potato Cultivars Assessed By In Vitro Screening Tala Khrais 1, Y. Leclerc 2, and Danielle J. Donnelly3 ~Graduate student, 3Associate Professor., Plant Science Department, Macdonald Campus, McGill University, 21 111 Lakeshore Road, Ste-Anne-de-Bellevue, QC, Canada H9X 3V9. -~Potato Physiologist. Potato Centre, New Brunswick Department of Agriculture, FlorenceviUe, NB, Canada E0J 1K0. Please send all correspondence to: Danielle J. Donnelly, Plant Science Department, Macdonald Campus, McGill University, 21,111 Lakeshore Road, Ste-Anne-de-Bellevue, Quebec, Canada H9X 3V9, phone: (514) 398-7851 (ext. 7856#), fax: (514) 398-7897, e-mail: [email protected]. ABSTRACT imiento (longitud de las ralces y de los brotes, peso fresco y seco) en la cosecha y se corrigieron debido a One hundred and thirty European and North Amer- sus diferencias en el vigor de la planta. Estos valores ican potato cultivars were assayed in vitro for salinity relativos fueron sujetos a un awl]i~is de multivariaci6n (NaC1) tolerance. A modified single-node cutting bioas- de grupos. La suma de los rangos relativos a 40, 80 y say was used in which cultivars were exposed to a 120 mM NaCI separaron los cultivares en 8 unidades. range of NaC1 levels (0, 40, 80, and 120 mM), in a Los cultivares Amisk, Belrus, Bintje, Onaway, Sierra y Murashige and Skoog-based medium, for 1 month. Eval- Tobique estuvieron en la unidad con mayor tolerancia uations were performed twice for each cultivar at each a la salinidad yen el grupo con mayor vigor, con excep- salt level, using five single-node cuttings. -

Double-Certified Organic Seed Potatoes
Double-Certified Organic Seed Potatoes Based in Idaho, Grand Teton Organics came into being 8 years ago after owner John Hoggan purchased the land from Parkinson Seed Farm. John has over 50 years of potato growing experience ranging from production, seed selection, plant breeding, seed stock certification and research & development of new varieties. In his career, he has grown approximately 500 different varieties of potatoes. For 2018, Country Farm & Home Supply will offer over 30 of Grand Teton Organics varieties. When your state’s motto is ‘The Potato State’, you can rest assured that Idaho’s Department of Agriculture takes growing potatoes to eat or to replant very seriously. Stringent protocol to ensure seed stock is top quality and as free of disease as possible are of the utmost importance to the state of Idaho and to Grand Teton Organics. Field inspections throughout the growing season, lab testing of seed stock and a winter crop of seed potatoes grown in Hawaii all ensure that your seed potatoes are the healthiest, most productive seed potatoes available. If at any time any of the seed potato lots begin to show signs of disease, they are culled and not made available for sale to ensure that overall disease presence in potato production areas (yours or theirs!) stays minimal and manageable. Grand Teton Organics is committed to providing the highest quality organic certified seed potatoes available on the market. Potatoes for seed start as tissue cultures from one of many Potato Germplasm Banks where parent stock is housed. Those tissues are then sent Idaho State University where they are grown out in labs to produce clean plants that are as disease-free as possible. -

Seed Potatoes You Would Like to Request from the Plant Materials Center (PMC) to Plant on Your Farm in 2019
5310 S. Bodenburg Spur Palmer, Alaska 99645-7646 Main: 907.745.4469 Fax: 907.746-1568 November 30, 2017 Dear Grower, Please consider the varieties and quantities of generation zero (G0) seed potatoes you would like to request from the Plant Materials Center (PMC) to plant on your farm in 2019. In order to establish our greenhouse production plans for 2018 at the PMC, we will accept order requests through January 31, 2018. We encourage you to renew your seed stocks as often as possible with disease free seed from the PMC to maintain high quality seed in Alaska potato production. In this regard, we are here to serve you and provide the industry with a healthy start. Please review the attached list and visit http://plants.alaska.gov/PotatoSeedProduction.html for ordering information. If you do not see a variety on the list that interests you, please contact us to see if we can produce the variety or a similar one. Based on production logistics, we are setting a minimum order limit of two pounds per variety. The price per pound is $15.00. Please do not hesitate to contact me with any questions. Sincerely, Christine Macknicki Potato Program Technician (907) 745-8021 [email protected] Available Public Varieties 8-3(Magic Myrna) Butte Favorite Red Maris Piper Rosa 22-1(Delta Red) Cal White French Fingerling Mark Varshaw Rose Finn Apple 29-6 (Fiesta) CalWhite Russet Frontier Russet Mirton Pearl Rose Gold AC Red Island Candy Cane German Butterball Mrs. Moehlers Yellow Royal Kidney AK114 Candystripe Goldrush Myatts Ashleaf Russet Burbank -

Seed Potato Info Only
The Villager Nursery 2010 Potatoes, Asparagus and Onions Virus Free Seed Potato Selection Yukon Gold (ORGANIC) Early Potato This round tuber has smooth, thin yellow skin with pink coloring around shallow eyes and yellow flesh. It provides excellent buttery flavor when baked, boiled or made into salads or fries; too moist for hash browns. The best selling early variety is a moderate keeper, and is drought-tolerant. Excellent yields. Most requested by farmer’s wife! Russet - Norkotah Early Potato Developed by Dr. Robert Johansen, talented breeder of the Anoka. Uniform tubers, brown skin and white flesh. Cooks excellent like any russet. It is scab-resistant and provides dependable yields.. It is a good choice for a gardener with limited space. Similar to Russet Burbank but home grown Improved Russet tastes nothing like the store bought spuds. Dark Red Norland Early Potato This potato has deep red -– almost burgundy skin (with shallow eyes) and white flesh. It makes good potato salad, keeps well, has good disease resistance and moderate scab resistance. This old favorite is high yielding. The red skin fades to pink in storage! This is the earliest red of them all. This variety was developed at the University of North Dakota for northern areas. Kennebec Mid-Season Potato Salt-of-the-earth kind of spud - never fails! Fine flavor. Boil or mash’em, peel’em or not. Smooth white skin, smooth-textured white flesh, shallow eyes make for easy washing and peeling. Resistant to mosaic, late blight and net necrosis. Dependably produces only large potatoes on most soils. Good keeper.