Report of a Working Group on Potato: First Meeting, 23-25 March 2000
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(12) Patent Application Publication (10) Pub. No.: US 2015/0259700 A1 Elling Et Al
US 2015025.9700A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2015/0259700 A1 Elling et al. (43) Pub. Date: Sep. 17, 2015 (54) TRANSGENC PLANTS WITH RNA Publication Classification INTERFERENCE-MEDIATED RESISTANCE AGAINST ROOT-KNOT NEMATODES (51) Int. Cl. CI2N 5/82 (2006.01) (71) Applicant: WASHINGTON STATE CI2N IS/II3 (2006.01) UNIVERSITY, PULLMAN, WA (US) (52) U.S. Cl. CPC .......... CI2N 15/8285 (2013.01); C12N 15/I 13 (72) Inventors: Axel A. Elling, Pullman, WA (US); (2013.01); C12N 23 10/141 (2013.01); C12N Charles R. Brown, Pullman, WA (US) 2310/531 (2013.01) (21) Appl. No.: 14/626,070 (57) ABSTRACT Transgenic plants that are stably resistant to the nematode (22) Filed: Feb. 19, 2015 Meloidogyne Chitwoodi are provided, as are methods of mak ing such transgenic plants. The transgenic plants (such as Related U.S. Application Data potatoes) are genetically engineered to express interfering (60) Provisional application No. 61/948,761, filed on Mar. RNA that targets the Meloidogyn effector protein 6, 2014. Mc16D1OL. Patent Application Publication US 2015/025.9700 A1 OIGI9I?JÄI TOIGI9IDJÄI OIGI9I?IAI TOICI9IDJÄI OIC19I?IN TOICI9IDWI Patent Application Publication Sep. 17, 2015 Sheet 2 of 11 US 2015/025.9700 A1 e h; Figure 2A Figure 2B Figure 2C Figure 2D Figure 3 Patent Application Publication Sep. 17, 2015 Sheet 3 of 11 US 2015/025.9700 A1 COL E2 D1 D2 D4 COL E2 D1 D2 D4 Figure 4A Figure 4B 25000 ;20000 15000 s 10000 2 5000 DES E29 D54 D56 D57 DES E29 D54 D56 D57 Figure 5A Figure 5B 60 1800 50 3. -

US20200383331A1.Pdf
US 20200383331A1 IN (19United States ( 12 ) Patent Application Publication ( Pub. No.:USQO2Q/QZ8333l Al HEINRICHER ( 43 ) Pub . Date : Dec. 10 , 2020 ( 54 ) COMPOSITIONS AND METHODS FOR AOIN 43/40 ( WQOQI LARGE - SCALE IN VITRO PLANT AOIN 43/08 ( 2006.01 ) BIOCULTURE A01N 37/52 ( 2006.01 ) AOIN 4730 ( 2006.01 ) ( II ) Applicant: BQOSHIQOQT LLC , Hailey, IDUS A016 22/15 ( WQGOI ( 52 ) U.S. CI . ( 72 ) Inventor: Jackie HEINRICHER , Anacortes , WA CPC AOIN 43/90 ( 2013.01 ) ; AO1G 31/00 (US ) ( 2013.01 ) ; A01N 59/08 ( 2013.01 ) ; A01N 59/20 ( 2013.01 ) ; A01N 59/16 ( 2013.01 ) ; ( 21 ) Appl . No .: 16 /728,478 A01N 59/14 ( 2013.01 ) ; A01N 31/06 ( 2013.01 ) ; A01N 43/78 ( 2013.01 ) ; A01N ( 22 ) Filed : Dec. 27 , 2019 37/10 ( 2013.01 ) ; A01N 43/82 ( 2013.01 ) ; AOIN 59/12 ( 2013.01 ) ; AOIN 37/44 Related U.S. Application Data ( 2013.01 ) ; A01N 43/40 ( 2013.01 ) ; A01N ( 63 ) Continuation of application No. PCT /US2018 / 43/08 ( 2013.01 ) ; A01N 37/52 ( 2013.01 ) ; 040637 , filed on Jul. 2 , 2018 , Continuation of appli AOIN 47/30 ( 2013.01 ) ; A01G 22/15 cation No. PCT/ US2018 / 040646 , filed on Jul. 2 , ( 2018.02 ) ; A01N 59/00 ( 2013.01 ) 2018 . ( 60 ) Provisional application No. 62 / 527,946 , filed on Jun . ( 57 ) ABSTRACT 3Q , provisional application No. 62 /6II , & a , The present invention provides media , kits , systems , and filed on Dec. 29 , 2017 , provisional application No. methods for achieving large scale pistachio production 62 / 527,862 , filed on Jun . 30 , 2017 . within a short time via bioculture , large scale yam produc tion within a short time via bioculture, high multiplication Publication Classification rate of plants including cannabis via in vitro micropropaga ( 51 ) Int . -

International Union for the Protection of New Varieties of Plants Geneva
E TG/23/6 ORIGINAL: English DATE: 2004-03-31 INTERNATIONAL UNION FOR THE PROTECTION OF NEW VARIETIES OF PLANTS GENEVA * POTATO (Solanum tuberosum L.) GUIDELINES FOR THE CONDUCT OF TESTS FOR DISTINCTNESS, UNIFORMITY AND STABILITY Alternative Names: * Latin English French German Spanish Solanum tuberosum L., Potato Pomme de terre Kartoffel Papa, Patata S. tuberosum L. sensu lato ASSOCIATED DOCUMENTS These guidelines should be read in conjunction with document TG/1/3, “G eneral Introduction to the Examination of Distinctness, Uniformity and Stability and the Development of Harmonized Descriptions of New Varieties of Plants” (hereinafter referred to as the “General Introduction”) and its associated “TGP” documents. * These names were correct at the time of the introduction of these Test Guidelines but may be revised or updated. [Readers are advised to consult the UPOV Code, which can be found on the UPOV Website (www.upov.int), for the latest infor mation.] TG/23/6 Potato, 2004 -03 -31 - 2 - TABLE OF CONTENTS 1. SUBJECT OF THESE TES T GUIDELINES ................................ ................................ ................................ .. 3 2. MATERIAL REQUIRED ................................ ................................ ................................ ............................... 3 3. METHOD OF EXAMINATIO N................................ ................................ ................................ ..................... 3 3.1 Duration of Tests ................................ ................................ ............................... -

Potato - Wikipedia, the Free Encyclopedia
Potato - Wikipedia, the free encyclopedia Log in / create account Article Talk Read View source View history Our updated Terms of Use will become effective on May 25, 2012. Find out more. Main page Potato Contents From Wikipedia, the free encyclopedia Featured content Current events "Irish potato" redirects here. For the confectionery, see Irish potato candy. Random article For other uses, see Potato (disambiguation). Donate to Wikipedia The potato is a starchy, tuberous crop from the perennial Solanum tuberosum Interaction of the Solanaceae family (also known as the nightshades). The word potato may Potato Help refer to the plant itself as well as the edible tuber. In the region of the Andes, About Wikipedia there are some other closely related cultivated potato species. Potatoes were Community portal first introduced outside the Andes region four centuries ago, and have become Recent changes an integral part of much of the world's cuisine. It is the world's fourth-largest Contact Wikipedia food crop, following rice, wheat and maize.[1] Long-term storage of potatoes Toolbox requires specialised care in cold warehouses.[2] Print/export Wild potato species occur throughout the Americas, from the United States to [3] Uruguay. The potato was originally believed to have been domesticated Potato cultivars appear in a huge variety of [4] Languages independently in multiple locations, but later genetic testing of the wide variety colors, shapes, and sizes Afrikaans of cultivars and wild species proved a single origin for potatoes in the area -

Tesis Doctoral 2017
MEJORA GENÉTICA DE PATATA PARA COMPUESTOS BIOACTIVOS Y CAPACIDAD ANTIOXIDANTE Roberto Tierno Fernández Tesis doctoral 2017 MEJORA GENÉTICA DE PATATA PARA COMPUESTOS BIOACTIVOS Y CAPACIDAD ANTIOXIDANTE Roberto Tierno Fernández Tesis doctoral Director: Dr. D. José Ignacio Ruiz de Galarreta Tutora: Dra. Dª. Mª Teresa Lacuesta Calvo (c)2017 ROBERTO TIERNO FERNANDEZ AGRADECIMIENTOS Este trabajo no hubiera sido posible sin la participación de muchos compañeros que, en mayor o menor medida, han contribuido a la realización de esta tesis. En primer lugar, mi reconocimiento al director de tesis, José Ignacio Ruiz de Galarreta, que apostó por mí desde el primer momento y durante este tiempo, me ha dado toda la confianza, libertad y apoyo que he necesitado. A la dirección de Neiker, por haberme acogido durante estos cuatro años y al INIA, organismo que financió este proyecto y posibilitó mi formación. Al Departamento de Biología Vegetal y Ecología de la UPV-EHU, por permitirme presentar este trabajo y especialmente a la Dra. Mª Teresa Lacuesta Calvo por aceptar ser tutora de esta tesis. Quiero agradecer la paciencia y buena disposición de muchas personas sin cuyas enseñanzas y consejos este trabajo jamás hubiera podido realizarse. Entre ellas, quiero hacer una mención muy especial a Isi, Carlos Castaño, Carlos Herrán y Bego. Otras personas con las que he tenido la oportunidad de trabajar y formarme son Ainara, Carmen, Silvia, Patrick Riga, Leire, Berdaitz y Jon. Por supuesto, quiero y debo reconocer también el inestimable aporte de Néstor, Raquel López, Mikel González, Emma López de Armentia, Jon Lemos y otros camaradas becarios, cuya guía, consejo y conocimiento ha sido determinante. -

Cartoful În România”
REDACğIA REVISTEI „CARTOFUL ÎN ROMÂNIA” CARTOFUL Institutul NaĠional de Cercetare-Dezvoltare pentru Cartof úi Sfeclă de Zahăr Braúov în România PublicaĦie de informare tehnicĆ pentru cultivatorii de cartof Adresa : 550470 Braúov, str. Fundăturii nr.2 Tel. 0268-476795, Fax 0268-476608 Volumul 20 Nr. 1, 2 2011 E-mail: [email protected] CUPRINS Web: www.potato.ro RUBRICA SPECIALISTULUI Colectivul de redacĠie: Dr.ing. Sorin CHIRU - TendinĠe actuale în procesarea cartofului Dr.ing. Victor DONESCU - Cartoful – materie primă pentru industrializare Ing. Gheorghe OLTEANU - Scurtă “pledoarie” pentru amidonul din cartof - Bune practici agricole la cultura cartofului industrial Drd.ing. Isabela PUIU - Rezultate ale cercetărilor privind procesarea cartofului Ing. Adrian GHINEA - ProtecĠia culturilor de cartof destinat prelucrării industriale - Biologia úi ecologia gândacului din Colorado, omniprezentul dăunător al culturilor de cartof - Metodele biologice, perspective moderne pentru controlul dăunătorilor din FederaĠia NaĠională Cartoful din România culturile de cartof - ParticularităĠi în păstrarea cartofului destinat prelucrării industriale Adresa: Hărman, str. Gări nr. 60B, 507085 - ÎnmulĠirea in vitro a soiurilor româneúti de cartof destinate procesării - Comportarea soiurilor de cartof pretabile la prelucrarea industrială, în Tel.0722-354913,Tel/Fax 0268-367551, 0268-368218 procesul de obĠinere a minituberculilor la INCDCSZ Braúov E-mail: [email protected] [email protected] - Pretabilitatea pentru industrializare a unor genotipuri de cartof obĠinute din Web: www.potato.ro/ro/fncr.php sămânĠă botanică Cod fiscal: 773969. Cont: RO05RZBR0000060000739734 - Modificări ale metodei de testare virotică a cartofului prin tehnica ELISA – limite, performanĠe úi avantaje pentru cartoful destinat industrializării Preúedinte: Ing. Ioan BENEA SIMPOZIONUL ZIUA VERDE A CARTOFULUI – 2011 - SituaĠia actuală a agriculturii judeĠului Covasna - Prezentarea activităĠii de cercetare la S.C.D.C. -
Seed Potato Directory 2017
The farm operation grows 93 acres of field generations one and two seed, operates 4 greenhouses producing conventional and NFT minitubers. Our stewardship of this seed continues through WISCONSIN the certification Our of stewardship these seed oflots this on seed Wisconsin continues seed through grower t farms, there is no other program like it. CERTIFIED The program maintains variety trueness to type; selecting and testing clones, rogueing of weak, genetic variants, and diseased plants to continue to develop and maintain germplasm of your SEED POTATOES favorite varieties at our laboratory. 103 Years of Seed Growing Tradition A Century Long Tradition Pioneers In Seed Potato Certification Administered since inception by the College of Agricultural and Life Sciences, University of Wisconsin – Madison, the program Much of the early research work on potato diseases and how retains a full-time staff of experienced professionals to ensure they spread was done Scientists in Germany found and that, Holland through around careful the monitoring turn thoroughness and impartiality in inspection and certification of the century. Scientists found that, through careful monitoring procedures. o of the crop and removal of unhealthy plants, Similar they could research maintain soon was a vigorous, healthy stock indefinitely. Similar research soon was Through providing information, exercising technical skill, doing b being conducted in the United States. research directed at solving problems, and conducting outreach activities, the University meets the growers at the field level. USDA plant pathologist W.A. Orton had studied potato This special relationship to the academic community brings new certification in Germany and upon his return, began to work with T information on pathogens, best practices, and introduces high potato growers and Universities to introduce those concepts quality basic seed into the marketplace. -

Foodservice Product Guide Draft
Page 1 Seasonal Calendar Seasons subject to weather Code Product Description Usual Case Size Other Units of Sale Special Notes Storage Temp Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec = usually available UK = UK season available = not available Please note seasons can vary year to year FRUIT APPBRAE Apple Braeburn Box (12.5kg) kg, each C UK UK UK UK UK APPBRAM Apple Bramley Box (12.5kg) kg, each C UK UK UK UK UK UK UK UK UK UK UK UK APPCOX Apple Coxes Box (12.5kg) kg, each C UK UK UK UK UK UK UK UK APPDIS Apple Discovery Box (12.5kg) kg, each C UK UK APPDELE Apple Delbard Estive Box (12.5kg) kg, each C UK UK UK APPLEJFUJI Apple Fuji Box (12.5kg) kg, each 24hr notice C APPGD Apple Golden Delicious Box (12.5kg) kg, each C APPGS Apple Granny Smith Box (18kg / 12.5kg) kg, each C APPJAZ Apple Jazz Box (12.5kg) kg, each C UK UK UK UK UK UK APPPL Apple Pink Lady Box (12.5kg) kg, each C APPRD Apple Red Delicious Box (18kg / 12.5kg) kg, each C APPREDW Apple Red Washington Box (12.5kg) kg, each C APPRG Applw Royal Gala Box (12.5kg) kg, each C UK UK UK UK UK UK UK APPRUS Apple Russet Box (12.5kg) kg, each C UK UK UK UK UK UK UK APPCRAB Apple Crab 500g punnet 24hr notice C UK UK APPCUS Apples Custard Box (12kg) box only 24hr notice C APRI Apricots Box (5kg) kg, each C AVOH Avocado Haas Box (Count 16) each C BABYKIW Baby Kiwi 125g punnet 24hours C BANB Baby Banana Box (3kg) kg, bunches C PLANTGR Banana Green Plantain Box (15kg) kg min order 5kg C BANL Banana Leaves 2 x 500g 500g packet C BAN Banana Med/Large Box (18kg) kg, each A BUDHA Budhas -

Potatoes in Practice 2012 Event Guide
3RWDWRHV3RWDWRHVPotatoes LQ3UDFWLFHLQ3UDFWLFHin Practice 2012 ThursdayThursday 12th 12th9th August August 9.30am9.30am to to 4.30pm 4.30pm4.30pm BalrudderyBalruddery Farm Farm InvergowrieInvergowrieInvergowrie DundeeDundee DD2 DD2DD2 5LJ 5LJ5LJ SupportedSupported by by FieldField Trials Trials & && Demonstrations, Demonstrations,Demonstrations, SeminarsSeminars and and Exhibitors Exhibitors Guide Guide ProgrammeContents Welcome to Potatoes in Practice 2012 Welcome to Potatoes in Practice 2012.................................................... 1 The James Hutton Institute, SAC, Agrii (formerly Masstock Arable) and the Potato Programme ................................................................................................ 2 Council welcome you to Potatoes in Practice 2012. This event is Britain’s premier field based event dedicated to the potato industry, attracting more international Site Plan ..................................................................................................... 4 visitors each year. Seminars .................................................................................................... 6 This is the fourth year PiP has been held at the James Hutton Institute’s Balruddery Farm. The event is supported by Potato Review. Exhibitor List .............................................................................................. 8 This is a unique opportunity for farmers, advisers and others to view government and industry- Field Trials and Demonstrations ........................................................... -
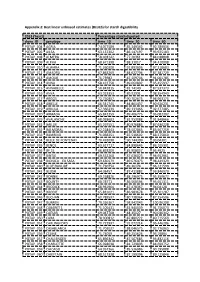
Percentage Starch Digested Clone ID
Appendix 2: Best linear unbiased estimates (BLUES) for starch digestibility 2013 (Year1) Percentage starch digested clone_ID genotype time_10 time_20 time_60 POTAP_004 AGRIA 74.077589 85.349342 92.789936 POTAP_005 AILSA 63.137302 86.147197 93.925183 POTAP_008 ALMERA 58.028142 77.20361 84.038808 POTAP_009 ALPHA 68.671398 88.31017 94.410008 POTAP_010 ALWARA 71.260208 92.893806 97.218683 POTAP_011 AMADRA 67.863269 84.622794 87.381729 POTAP_013 AMOUR 71.73982 87.109706 91.001785 POTAP_014 ANNA 68.422758 89.660892 95.455937 POTAP_015 ANNABELLE 58.843815 82.74149 87.537373 POTAP_016 ANYA 63.923353 89.92991 98.354949 POTAP_017 ARGOS 72.750357 90.702675 98.963175 POTAP_018 ARIELLE 66.341745 84.861437 90.926231 POTAP_019 ARKULA 67.366239 89.333466 92.377485 POTAP_020 ARMADA 65.842359 89.406775 95.082302 POTAP_029 AVALANCHE 68.038683 81.953088 91.848666 POTAP_031 BAILLIE 65.297551 80.976885 87.636228 POTAP_032 BALMORAL 62.228443 78.502863 83.567259 POTAP_033 BAMBINO 71.038251 87.518602 88.375869 POTAP_034 BELLE DE FONTENAY 70.492098 90.744399 93.04301 POTAP_035 BENOL 63.617717 84.898645 86.105301 POTAP_036 BF 15 66.893209 85.908057 93.4925 POTAP_037 BINTJE 69.532268 87.636023 94.952961 POTAP_038 BIONICA EX SASA 69.630179 89.676473 98.449329 POTAP_040 BLUE DANUBE 71.730754 89.36174 96.121599 POTAP_041 BLUSH 64.03457 87.411882 93.846979 POTAP_042 BONNIE 69.258826 88.296975 95.359672 POTAP_043 BOUNTY 68.78771 86.568594 95.651311 POTAP_044 BRODICK 68.961964 87.478367 94.04128 POTAP_045 BRODIE 65.831021 86.983678 95.301287 POTAP_046 BUCHAN 60.789597 -

Relative Salinity Tolerance of Potato Cultivars Assessed by <Emphasis Type="Italic">In Vitro </Emphasis> S
Amer J of Potato Res (1998) 75:207-210 207 Relative Salinity Tolerance of Potato Cultivars Assessed By In Vitro Screening Tala Khrais 1, Y. Leclerc 2, and Danielle J. Donnelly3 ~Graduate student, 3Associate Professor., Plant Science Department, Macdonald Campus, McGill University, 21 111 Lakeshore Road, Ste-Anne-de-Bellevue, QC, Canada H9X 3V9. -~Potato Physiologist. Potato Centre, New Brunswick Department of Agriculture, FlorenceviUe, NB, Canada E0J 1K0. Please send all correspondence to: Danielle J. Donnelly, Plant Science Department, Macdonald Campus, McGill University, 21,111 Lakeshore Road, Ste-Anne-de-Bellevue, Quebec, Canada H9X 3V9, phone: (514) 398-7851 (ext. 7856#), fax: (514) 398-7897, e-mail: [email protected]. ABSTRACT imiento (longitud de las ralces y de los brotes, peso fresco y seco) en la cosecha y se corrigieron debido a One hundred and thirty European and North Amer- sus diferencias en el vigor de la planta. Estos valores ican potato cultivars were assayed in vitro for salinity relativos fueron sujetos a un awl]i~is de multivariaci6n (NaC1) tolerance. A modified single-node cutting bioas- de grupos. La suma de los rangos relativos a 40, 80 y say was used in which cultivars were exposed to a 120 mM NaCI separaron los cultivares en 8 unidades. range of NaC1 levels (0, 40, 80, and 120 mM), in a Los cultivares Amisk, Belrus, Bintje, Onaway, Sierra y Murashige and Skoog-based medium, for 1 month. Eval- Tobique estuvieron en la unidad con mayor tolerancia uations were performed twice for each cultivar at each a la salinidad yen el grupo con mayor vigor, con excep- salt level, using five single-node cuttings. -

The Royal Horticultural Society
The Royal Horticultural Society Vegetable Seed Suppliers Listing 2010 TO USE THIS DOCUMENT: 1. Click on + next to CROPS in the sidebar to select vegetable type 2. Click on + next to SUPPLIERS in the sidebar for a key to the numeric supplier codes The RHS, the UK’s leading gardening charity Vegetable Seed Suppliers 2010 © The Royal Horticultural Society Crop Cultivar name as listed in catalogue Standard seed suppliers Organic seed suppliers Grand Total ADZUKI BEANS ADZUKI BEANS 1 1/351 Vegetable Seed Suppliers 2010 © The Royal Horticultural Society Crop Cultivar name as listed in catalogue Standard seed suppliers Organic seed suppliers ALFALFA Total ALFALFA ALFALFA 1•3•4•6•7•9•13•14•20•21•30•34 12•15•17•31 2/351 Vegetable Seed Suppliers 2010 © The Royal Horticultural Society Crop Cultivar name as listed in catalogue Standard seed suppliers Organic seed suppliers ARTICHOKE Total ARTICHOKE CHINESE 6•7•8•10•11•12 15 ARTICHOKE CONCERTO (H) 7•10•35 ARTICHOKE EMERALD 1•6•9 ARTICHOKE GREEN GLOBE 1•2•3•5•7•8•9•10•11•13•14•15•18•20•21•22•25•30•34•36 12 ARTICHOKE GROS VERT DE LAON 4•36 ARTICHOKE LARGE GREEN PARIS 25 ARTICHOKE ORIGINALS/COMMON 10•11 ARTICHOKE PURPLE GLOBE 1•12•14•21•25•31 ARTICHOKE ROMANESCO 1•2•4•15•18•20•22 ARTICHOKE VIOLET GLOBE 8 ARTICHOKE VIOLETTE DE PROVENCE 1•14•34 ARTICHOKE VIOLETTO DI CHOGGIA 1•5•13•22•27 ARTICHOKE IMPERIAL STAR 1•32 15•17•25•31 3/351 Vegetable Seed Suppliers 2010 © The Royal Horticultural Society Crop Cultivar name as listed in catalogue Standard seed suppliers Organic seed suppliers ARTICHOKE - JERUSALEM