On the Rat Trail in Near Oceania: Applying the Commensal Model to the Question of the Lapita Colonization1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Archaeology of Lapita Dispersal in Oceania
The archaeology of Lapita dispersal in Oceania pers from the Fourth Lapita Conference, June 2000, Canberra, Australia / Terra Australis reports the results of archaeological and related research within the south and east of Asia, though mainly Australia, New Guinea and Island Melanesia — lands that remained terra australis incognita to generations of prehistorians. Its subject is the settlement of the diverse environments in this isolated quarter of the globe by peoples who have maintained their discrete and traditional ways of life into the recent recorded or remembered past and at times into the observable present. Since the beginning of the series, the basic colour on the spine and cover has distinguished the regional distribution of topics, as follows: ochre for Australia, green for New Guinea, red for Southeast Asia and blue for the Pacific islands. From 2001, issues with a gold spine will include conference proceedings, edited papers, and monographs which in topic or desired format do not fit easily within the original arrangements. All volumes are numbered within the same series. List of volumes in Terra Australis Volume 1: Burrill Lake and Currarong: coastal sites in southern New South Wales. R.J. Lampert (1971) Volume 2: Ol Tumbuna: archaeological excavations in the eastern central Highlands, Papua New Guinea. J.P. White (1972) Volume 3: New Guinea Stone Age Trade: the geography and ecology of traffic in the interior. I. Hughes (1977) Volume 4: Recent Prehistory in Southeast Papua. B. Egloff (1979) Volume 5: The Great Kartan Mystery. R. Lampert (1981) Volume 6: Early Man in North Queensland: art and archeaology in the Laura area. -

Biodiversity of the Kermadec Islands and Offshore Waters of the Kermadec Ridge: Report of a Coastal, Marine Mammal and Deep-Sea Survey (TAN1612)
Biodiversity of the Kermadec Islands and offshore waters of the Kermadec Ridge: report of a coastal, marine mammal and deep-sea survey (TAN1612) New Zealand Aquatic Environment and Biodiversity Report No. 179 Clark, M.R.; Trnski, T.; Constantine, R.; Aguirre, J.D.; Barker, J.; Betty, E.; Bowden, D.A.; Connell, A.; Duffy, C.; George, S.; Hannam, S.; Liggins, L..; Middleton, C.; Mills, S.; Pallentin, A.; Riekkola, L.; Sampey, A.; Sewell, M.; Spong, K.; Stewart, A.; Stewart, R.; Struthers, C.; van Oosterom, L. ISSN 1179-6480 (online) ISSN 1176-9440 (print) ISBN 978-1-77665-481-9 (online) ISBN 978-1-77665-482-6 (print) January 2017 Requests for further copies should be directed to: Publications Logistics Officer Ministry for Primary Industries PO Box 2526 WELLINGTON 6140 Email: [email protected] Telephone: 0800 00 83 33 Facsimile: 04-894 0300 This publication is also available on the Ministry for Primary Industries websites at: http://www.mpi.govt.nz/news-resources/publications.aspx http://fs.fish.govt.nz go to Document library/Research reports © Crown Copyright - Ministry for Primary Industries TABLE OF CONTENTS EXECUTIVE SUMMARY 1 1. INTRODUCTION 3 1.1 Objectives: 3 1.2 Objective 1: Benthic offshore biodiversity 3 1.3 Objective 2: Marine mammal research 4 1.4 Objective 3: Coastal biodiversity and connectivity 5 2. METHODS 5 2.1 Survey area 5 2.2 Survey design 6 Offshore Biodiversity 6 Marine mammal sampling 8 Coastal survey 8 Station recording 8 2.3 Sampling operations 8 Multibeam mapping 8 Photographic transect survey 9 Fish and Invertebrate sampling 9 Plankton sampling 11 Catch processing 11 Environmental sampling 12 Marine mammal sampling 12 Dive sampling operations 12 Outreach 13 3. -

Late Holocene Human Expansion Into Near and Remote Oceania: a Bayesian Model of the Chronologies of the Mariana Islands and Bismarck Archipelago
The Journal of Island and Coastal Archaeology ISSN: 1556-4894 (Print) 1556-1828 (Online) Journal homepage: http://www.tandfonline.com/loi/uica20 Late Holocene Human Expansion into Near and Remote Oceania: A Bayesian Model of the Chronologies of the Mariana Islands and Bismarck Archipelago Timothy M. Rieth & J. Stephen Athens To cite this article: Timothy M. Rieth & J. Stephen Athens (2017): Late Holocene Human Expansion into Near and Remote Oceania: A Bayesian Model of the Chronologies of the Mariana Islands and Bismarck Archipelago, The Journal of Island and Coastal Archaeology, DOI: 10.1080/15564894.2017.1331939 To link to this article: http://dx.doi.org/10.1080/15564894.2017.1331939 View supplementary material Published online: 07 Jun 2017. Submit your article to this journal View related articles View Crossmark data Full Terms & Conditions of access and use can be found at http://www.tandfonline.com/action/journalInformation?journalCode=uica20 Download by: [66.66.217.214] Date: 07 June 2017, At: 09:52 The Journal of Island and Coastal Archaeology, 0:1–12, 2017 Copyright C Taylor & Francis Group, LLC ISSN: 1556-4894 print / 1556-1828 online DOI: 10.1080/15564894.2017.1331939 Late Holocene Human Expansion into Near and Remote Oceania: A Bayesian Model of the Chronologies of the Mariana Islands and Bismarck Archipelago Timothy M. Rieth and J. Stephen Athens International Archaeological Research Institute, Inc., Honolulu, Hawaii, USA ABSTRACT Since the investigations of Spoehr in the 1950s, most researchers have accepted a date of ∼3500 BP/1500 BC for the initial human settle- ment of the Mariana Islands in the western Pacific. -

My Friend, the Volcano (Adapted from the 2004 Submarine Ring of Fire Expedition)
New Zealand American Submarine Ring of Fire 2007 My Friend, The Volcano (adapted from the 2004 Submarine Ring of Fire Expedition) FOCUS MAXIMUM NUMBER OF STUDENTS Ecological impacts of volcanism in the Mariana 30 and Kermadec Islands KEY WORDS GRADE LEVEL Ring of Fire 5-6 (Life Science/Earth Science) Asthenosphere Lithosphere FOCUS QUESTION Magma What are the ecological impacts of volcanic erup- Fault tions on tropical island arcs? Transform boundary Convergent boundary LEARNING OBJECTIVES Divergent boundary Students will be able to describe at least three Subduction beneficial impacts of volcanic activity on marine Tectonic plate ecosystems. BACKGROUND INFORMATION Students will be able to explain the overall tec- The Submarine Ring of Fire is an arc of active vol- tonic processes that cause volcanic activity along canoes that partially encircles the Pacific Ocean the Mariana Arc and Kermadec Arc. Basin, including the Kermadec and Mariana Islands in the western Pacific, the Aleutian Islands MATERIALS between the Pacific and Bering Sea, the Cascade Copies of “Marianas eruption killed Anatahan’s Mountains in western North America, and numer- corals,” one copy per student or student group ous volcanoes on the western coasts of Central (from http://www.cdnn.info/eco/e030920/e030920.html) America and South America. These volcanoes result from the motion of large pieces of the AUDIO/VISUAL MATERIALS Earth’s crust known as tectonic plates. None Tectonic plates are portions of the Earth’s outer TEACHING TIME crust (the lithosphere) about 5 km thick, as Two or three 45-minute class periods, plus time well as the upper 60 - 75 km of the underlying for student research mantle. -

The Bioarchaeology of Initial Human Settlement in Palau
THE BIOARCHAEOLOGY OF INITIAL HUMAN SETTLEMENT IN PALAU, WESTERN MICRONESIA by JESSICA H. STONE A DISSERTATION Presented to the Department of Anthropology and the Graduate School of the University of Oregon in partial fulfillment of the requirements for the degree of Doctor of Philosophy June 2020 DISSERTATION APPROVAL PAGE Student: Jessica H. Stone Title: The Bioarchaeology of Initial Human Settlement in Palau, Western Micronesia This dissertation has been accepted and approved in partial fulfillment of the requirements for the Doctor of Philosophy degree in the Department of Anthropology by: Scott M. Fitzpatrick Chairperson Nelson Ting Core Member Dennis H. O’Rourke Core Member Stephen R. Frost Core Member James Watkins Institutional Representative and Kate Mondloch Interim Vice Provost and Dean of the Graduate School Original approval signatures are on file with the University of Oregon Graduate School. Degree awarded June 2020 ii © 2020 Jessica H. Stone iii DISSERTATION ABSTRACT Jessica H. Stone Doctor of Philosophy Department of Anthropology June 2020 Title: The Bioarchaeology of Initial Human Settlement in Palau, Western Micronesia The initial settlement of Remote Oceania represents the world’s last major wave of human dispersal. While transdisciplinary models involving linguistic, archaeological, and biological data have been utilized in the Pacific to develop basic chronologies and trajectories of initial human settlement, a number of elusive gaps remain in our understanding of the region’s colonization history. This is especially true in Micronesia, where a paucity of human skeletal material dating to the earliest periods of settlement have hindered biological contributions to colonization models. The Chelechol ra Orrak site in Palau, western Micronesia, contains the largest and oldest human skeletal assemblage in the region, and is one of only two known sites that represent some of the earliest settlers in the Pacific. -
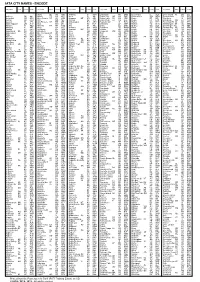
Iata City Names - Encode
IATA CITY NAMES - ENCODE City name State Country Code City name State Country Code City name State Country Code City name State Country Code City name State Country Code City name State Country Code Alpha QL AU ABH Aribinda BF XAR Bakelalan MY BKM Beersheba IL BEV Block Island RI US BID Aalborg DK AAL Alpine TX US ALE Arica CL ARI Baker City OR US BKE Befandriana MG WBD Bloemfontein ZA BFN Aalesund NO AES Alroy Downs NT AU AYD Aripuana MT BR AIR Baker Lake NU CA YBK Beica ET BEI Blonduos IS BLO Aarhus DK AAR Alta NO ALF Arkalyk KZ AYK Bakersfield CA US BFL Beida LY LAQ Bloodvein MB CA YDV Aasiaat GL JEG Alta Floresta MT BR AFL Arkhangelsk RU ARH Bakkafjordur IS BJD Beihai CN BHY Bloomfield Ri QL AU BFC Aba/Hongyuan CN AHJ Altai MN LTI Arlit NE RLT Bakouma CF BMF Beihan YE BHN Bloomington IN US BMG Abadan IR ABD Altamira PA BR ATM Arly BF ARL Baku AZ BAK Beijing CN BJS Bloomington-NIL US BMI Abaiang KI ABF Altay CN AAT Armenia CO AXM Balakovo RU BWO Beira MZ BEW Blubber Bay BC CA XBB Abakan XU ABA Altenburg DE AOC Armidale NS AU ARM Balalae SB BAS Beirut LB BEY Blue Bell PA US BBX Abbotsford BC CA YXX Altenrhein CH ACH Arno MH AMR Balgo Hill WA AU BQW Bejaia DZ BJA Bluefield WV US BLF Abbottabad PK AAW Alto Rio Seng CB AR ARR Aroa PG AOA Bali CM BLC Bekily MG OVA Bluefields NI BEF Abbs YE EAB Alton IL US ALN Arona SB RNA Bali PG BAJ Belaga MY BLG Blumenau SC BR BNU Abeche TD AEH Altoona PA US AOO Arorae KI AIS Balikesir TR BZI Belem PA BR BEL Blythe CA US BLH Abemama KI AEA Altus OK US LTS Arrabury QL AU AAB Balikpapan ID BPN Belfast GB -

Patterns of Prehistoric Human Mobility in Polynesia Indicated by Mtdna from the Pacific Rat (Rattus Exulans͞population Mobility)
Proc. Natl. Acad. Sci. USA Vol. 95, pp. 15145–15150, December 1998 Anthropology Patterns of prehistoric human mobility in Polynesia indicated by mtDNA from the Pacific rat (Rattus exulansypopulation mobility) E. MATISOO-SMITH*†,R.M.ROBERTS‡,G.J.IRWIN*, J. S. ALLEN*, D. PENNY§, AND D. M. LAMBERT¶ *Department of Anthropology and ‡School of Biological Sciences, University of Auckland, P. B. 92019 Auckland, New Zealand; and §Molecular Genetics Unit and ¶Department of Ecology, Massey University, P. B. 11222 Palmerston North, New Zealand Communicated by R. C. Green, University of Auckland, Auckland, New Zealand, October 14, 1998 (received for review July 20, 1998) ABSTRACT Human settlement of Polynesia was a major Recent genetic research focusing on Polynesian populations event in world prehistory. Despite the vastness of the distances has contributed significantly to our understanding of the covered, research suggests that prehistoric Polynesian popu- ultimate origins of this last major human migration. Studies of lations maintained spheres of continuing interaction for at globin gene variation (2) and mtDNA lineages of modern least some period of time in some regions. A low level of genetic Polynesians (3, 4) and studies of ancient DNA from Lapita- variation in ancestral Polynesian populations, genetic admix- associated skeletons (5) may indicate that some degree of ture (both prehistoric and post-European contact), and severe admixture with populations in Near Oceania occurred as more population crashes resulting from introduction of European remote biological ancestors left Southeast Asia and passed diseases make it difficult to trace prehistoric human mobility through Near Oceania. An alternative hypothesis is that the in the region by using only human genetic and morphological biological ancestors of these groups were one of a number of markers. -

Obsidian from Macauley Island: a New Zealand Connection
www.aucklandmuseum.com Obsidian from Macauley Island: a New Zealand connection Louise Furey Auckland War Memorial Museum Callan Ross-Sheppard McGill University, Montreal Kath E. Prickett Auckland War Memorial Museum Abstract An obsidian flake collected from Macauley Island during the Kermadec Expedition has been analysed to determine the source. The result indicates a Mayor Island, New Zealand, origin, supporting previous results on obsidian from Raoul Island that Polynesians travelled back into the Pacific from New Zealand. Keywords obsidian; Pacific colonisation; Pacific voyaging; Macauley Island. INTRODUCTION The steep terrain meant there were only a few suitable habitation places confined to flat areas of limited size on The Kermadec Islands comprise Raoul Island, the largest the south and north coasts. The only landing places were of the group, the nearby Meyer Islands and Herald adjacent to these flats. Botanical surveys have recorded Islets, and 100–155 km to the south are Macauley, the presence of Polynesian tropical cultigens including Curtis and Cheeseman iIslands, and L’Esperence Rock. candlenut (Aleurites monuccana) and ti (Cordyline Raoul is approximately 1000 km to the northeast of fruticosa) (Sykes 1977) which are not endemic to the New Zealand. During the 2011 Kermadec Expedition, island and it is assumed they were transported there by samples of naturally occurring obsidian were obtained Polynesian settlers. Polynesian tuberous vegetables such from Raoul, the Meyer Islands, and from the sea floor off as taro (Colocasia esculenta) and kumara (Ipomoea Curtis Island. These are now in the Auckland Museum batatas) were also recorded but their introduction collection. More importantly however was the single attributed to later 19th century immigrants. -

IATA Codes for Papua New Guinea
IATA Codes for Papua New Guinea N.B. To check the official, current database of IATA Codes see: http://www.iata.org/publications/Pages/code-search.aspx City State IATA Code Airport Name Web Address Afore AFR Afore Airstrip Agaun AUP Aiambak AIH Aiambak Aiome AIE Aiome Aitape ATP Aitape Aitape TAJ Tadji Aiyura Valley AYU Aiyura Alotau GUR Ama AMF Ama Amanab AMU Amanab Amazon Bay AZB Amboin AMG Amboin Amboin KRJ Karawari Airstrip Ambunti AUJ Ambunti Andekombe ADC Andakombe Angoram AGG Angoram Anguganak AKG Anguganak Annanberg AOB Annanberg April River APR April River Aragip ARP Arawa RAW Arawa City State IATA Code Airport Name Web Address Arona AON Arona Asapa APP Asapa Aseki AEK Aseki Asirim ASZ Asirim Atkamba Mission ABP Atkamba Aua Island AUI Aua Island Aumo AUV Aumo Babase Island MKN Malekolon Baimuru VMU Baindoung BDZ Baindoung Bainyik HYF Hayfields Balimo OPU Bambu BCP Bambu Bamu BMZ Bamu Bapi BPD Bapi Airstrip Bawan BWJ Bawan Bensbach BSP Bensbach Bewani BWP Bewani Bialla, Matalilu, Ewase BAA Bialla Biangabip BPK Biangabip Biaru BRP Biaru Biniguni XBN Biniguni Boang BOV Bodinumu BNM Bodinumu Bomai BMH Bomai Boridi BPB Boridi Bosset BOT Bosset Brahman BRH Brahman 2 City State IATA Code Airport Name Web Address Buin UBI Buin Buka BUA Buki FIN Finschhafen Bulolo BUL Bulolo Bundi BNT Bundi Bunsil BXZ Cape Gloucester CGC Cape Gloucester Cape Orford CPI Cape Rodney CPN Cape Rodney Cape Vogel CVL Castori Islets DOI Doini Chungribu CVB Chungribu Dabo DAO Dabo Dalbertis DLB Dalbertis Daru DAU Daup DAF Daup Debepare DBP Debepare Denglagu Mission -

Table of Contents
Commercial in Confidence Development Proposal Wind Emirau Sustainable economic growth for Papua New Guinea… …with a PNG difference Prepared by Edward Car Wind Australia PO Box 377 Kangaroo Ground Victoria, Australia 3097 Tel: 613 9712 0533 [email protected] www.windaustralia.com July 2004 © Copyright Wind Australia 2003 Commercial in Confidence Wind Emirau Project Table of Contents Executive Summary 3 Background 4 Drivers of change Proposal Overview 8 Objectives 15 Marketing Plan 16 Marketing Objectives 21 Pricing 25 SWOT 30 Implementation Plan 34 Operational Issues 39 Infrastructure Development 44 Benefits 53 Ownership 55 Government Assistance Required 58 Risks 60 Financials 61 Glossary 63 Appendix A Developing Sustainable Commercial Fisheries 64 B Project Location 80 C Fish Aggregating Devices (FAD) 83 D Tuna Exports 1996 – June 2001 84 E Assumptions – Summary 89 F Time to Market Scenario 91 2 Commercial in Confidence Wind Emirau Project Executive Summary An International airport and deep-sea port development on Emirau Island will provide a gateway for Papua New Guinea (PNG) to access international markets and export fresh tuna and seafood and establish the largest fresh tuna market in the world. The airport designed for Boeing 747 and the new Airbus 380 will also provide a staging point for control of the vast PNG Designated Fishing Zone (DFZ). Effectively this increases the level of regulation and compliance on visiting international fishing fleets and provides a way of monitoring their impact. Initially the project focus is on developing commercial artisanal fisheries for St Mathias islanders and marketing high quality hand-fished fresh seafoods under a unique brand that promotes the sustainability of the fisheries, the environment and a values- based society. -

A Rapid Biodiversity Survey of Papua New Guinea’S Manus and Mussau Islands
A Rapid Biodiversity Survey of Papua New Guinea’s Manus and Mussau Islands edited by Nathan Whitmore Published by: Wildlife Conservation Society Papua New Guinea Program PO BOX 277, Goroka, Eastern Highlands Province PAPUA NEW GUINEA Tel: +675-532-3494 www.wcs.org Editor: Nathan Whitmore. Authors: Ken P. Aplin, Arison Arihafa, Kyle N. Armstrong, Richard Cuthbert, Chris J. Müller, Junior Novera, Stephen J. Richards, William Tamarua, Günther Theischinger, Fanie Venter, and Nathan Whitmore. The Wildlife Conservation Society is a private, not-for-profit organisation exempt from federal income tax under section 501c(3) of the Inland Revenue Code. The opinions expressed in this publication are those of the contributors and do not necessarily reflect those of the Wildlife Conservation Society, the Criticial Ecosystems Partnership Fund, nor the Papua New Guinean Department of Environment or Conservation. Suggested citation: Whitmore N. (editor) 2015. A rapid biodiversity survey of Papua New Guinea’s Manus and Mussau Islands. Wildlife Conservation Society Papua New Guinea Program. Goroka, PNG. ISBN: 978-0-9943203-1-5 Front cover Image: Fanie Venter: cliffs of Mussau. ©2015 Wildlife Conservation Society A rapid biodiversity survey of Papua New Guinea’s Manus and Mussau Islands. Edited by Nathan Whitmore Table of Contents Participants i Acknowledgements iii Organisational profiles iv Letter of support v Foreword vi Executive summary vii Introduction 1 Chapters 1: Plants of Mussau Island 4 2: Butterflies of Mussau Island (Lepidoptera: Rhopalocera) -
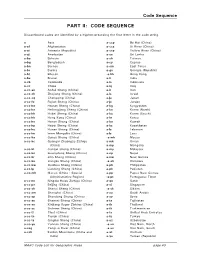
Code Sequence
Code Sequence PART II: CODE SEQUENCE Discontinued codes are identified by a hyphen preceding the first letter in the code string. a Asia a-ccp Bo Hai (China) a-af Afghanistan a-ccs Xi River (China) a-ai Armenia (Republic) a-ccy Yellow River (China) a-aj Azerbaijan a-ce Sri Lanka a-ba Bahrain a-ch Taiwan a-bg Bangladesh a-cy Cyprus a-bn Borneo a-em East Timor a-br Burma a-gs Georgia (Republic) a-bt Bhutan -a-hk Hong Kong a-bx Brunei a-ii India a-cb Cambodia a-io Indonesia a-cc China a-iq Iraq a-cc-an Anhui Sheng (China) a-ir Iran a-cc-ch Zhejiang Sheng (China) a-is Israel a-cc-cq Chongqing (China) a-ja Japan a-cc-fu Fujian Sheng (China) a-jo Jordan a-cc-ha Hainan Sheng (China) a-kg Kyrgyzstan a-cc-he Heilongjiang Sheng (China) a-kn Korea (North) a-cc-hh Hubei Sheng (China) a-ko Korea (South) a-cc-hk Hong Kong (China) a-kr Korea a-cc-ho Henan Sheng (China) a-ku Kuwait a-cc-hp Hebei Sheng (China) a-kz Kazakhstan a-cc-hu Hunan Sheng (China) a-le Lebanon a-cc-im Inner Mongolia (China) a-ls Laos a-cc-ka Gansu Sheng (China) -a-mh Macao a-cc-kc Guangxi Zhuangzu Zizhiqu a-mk Oman (China) a-mp Mongolia a-cc-ki Jiangxi Sheng (China) a-my Malaysia a-cc-kn Guangdong Sheng (China) a-np Nepal a-cc-kr Jilin Sheng (China) a-nw New Guinea a-cc-ku Jiangsu Sheng (China) -a-ok Okinawa a-cc-kw Guizhou Sheng (China) a-ph Philippines a-cc-lp Liaoning Sheng (China) a-pk Pakistan a-cc-mh Macao (China : Special a-pp Papua New Guinea Administrative Region) -a-pt Portuguese Timor a-cc-nn Ningxia Huizu Zizhiqu (China) a-qa Qatar a-cc-pe Beijing (China) a-si Singapore