Guidewire-Mounted Thermal Sensors to Assess Coronary Hemodynamics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

At May 2013 Proof All.Pdf
2013 SEASON PREVIEW — PAGES 6–7 Q&A WITH HERSCHEND’S JOEL MANEY — PAGES 41–42 © TM Your Amusement Industry NEWS Leader! Vol. 17 • Issue 2 MAY 2013 Merlin Entertainments’ U.S. Legoland Hotel a brickwork bonanza Southern California leap into the destination cat- their perspective that has gone egory. into the planning first and park becomes Officially opened April foremost.” full-fledged resort 5 after several days of me- AT found this in abundant dia previews, the three-story, evidence during a visit to the STORY: Dean Lamanna Special to Amusement Today 250-room inn, like the park, brightly multicolored hotel is designed to immerse fami- — beginning with the giant, CARLSBAD, Calf. — With lies with children aged two stream-breathing green drag- its unique toy theme and se- to 12 in the creative world of on made from some 400,000 ries of tasteful, steadfastly Lego toys. Guests of the hotel, Lego bricks that welcomes kid-focused additions over which is located adjacent to lodgers while guarding the its 14-year history, including Legoland’s entrance gate, will porte cochere from a clock an aquarium in 2008 and a have early-morning access to tower. Inside the lobby, which waterpark in 2010, Legoland the park of up to an hour be- contains a “wading pond” California established itself as fore the general public is ad- filled with Lego bricks, several a serious player in Southern mitted. of the more than 3,500 elabo- California’s heated amuse- “This is a one-of-a-kind rate Lego models adorning the ment market. -
Atletscorer Loaded 2012
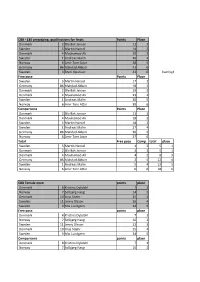
CBB - 180 prejudging, qualifications for finals Points Place Danmark 2 Ole Bak Jensen 11 1 Sweden 5 Martin Hanzel 14 2 Denmark 4 Mouhamad Ali 20 3 Sweden 1 Andreas Malm 26 4 Norway 6 Amir Tore Attar 38 5 Germany 86 Mahdad Akbari 43 6 Sweden 3 Brani Oparusic 43 6 had highest individual score Free pose Points Place Sweden 5 Martin Hanzel 17 1 Germany 86 Mahdad Akbari 18 2 Danmark 2 Ole Bak Jensen 19 3 Denmark 4 Mouhamad Ali 23 4 Sweden 1 Andreas Malm 30 5 Norway 6 Amir Tore Attar 39 6 Comparisons Points Place Danmark 2 Ole Bak Jensen 11 1 Denmark 4 Mouhamad Ali 18 2 Sweden 5 Martin Hanzel 18 2 Sweden 1 Andreas Malm 27 4 Germany 86 Mahdad Akbari 36 5 Norway 6 Amir Tore Attar 37 6 Total Free pose Comp. total place Sweden 5 Martin Hanzel 1 2 5 1 Danmark 2 Ole Bak Jensen 3 1 5 1 Denmark 4 Mouhamad Ali 4 2 8 3 Germany 86 Mahdad Akbari 2 5 12 4 Sweden 1 Andreas Malm 5 4 13 5 Norway 6 Amir Tore Attar 6 6 18 6 CBB Female open points place Denmark 8 Kristina Dybdahl 7 1 Norway 7 Solbjørg Haug 14 2 Denmark 10 Anja Stæhr 25 3 Sweden 11 Jenny Olsson 26 4 Sweden 9 Mia Lundgren 34 5 Free pose points place Denmark 8 Kristina Dybdahl 7 1 Norway 7 Solbjørg Haug 16 2 Sweden 11 Jenny Olsson 21 3 Denmark 10 Anja Stæhr 25 4 Sweden 9 Mia Lundgren 34 5 Comparisons points place Denmark 8 Kristina Dybdahl 7 1 Norway 7 Solbjørg Haug 16 2 Denmark 10 Anja Stæhr 22 3 Sweden 11 Jenny Olsson 25 4 Sweden 9 Mia Lundgren 34 5 total free posing comp. -
![HRP]SXP X] Cwt GPX]](https://docslib.b-cdn.net/cover/5278/hrp-sxp-x-cwt-gpx-1975278.webp)
HRP]SXP X] Cwt GPX]
FOLK DANCE SCENE First Class Mail 4362 COOLIDGE AVE. U.S. POSTAGE LOS ANGELES, CA 90066 PAID Culver City, CA Permit No. 69 First Class Mail Dated Material ORDER FORM Please enter my subscription to FOLK DANCE SCENE for one year, beginning with the next published issue. Subscription rate: $15.00/year (U.S. First Class), $18.00/year in U.S. currency (Foreign) Published monthly except for June/July and December/January issues. NAME _________________________________________ ADDRESS _________________________________________ PHONE (_____)_____–________ CITY _________________________________________ STATE __________________ E-MAIL _________________________________________ ZIP __________–________ Please mail subscription orders to the Subscription Office: 2010 Parnell Avenue Los Angeles, CA 90025 (Allow 6-8 weeks for subscription to go into effect if order is mailed after the 10th of the month.) Scandia in the Rain Published by the Folk Dance Federation of California, South Volume 38, No. 6 August 2002 Folk Dance Scene Committee Club Directory Coordinators Jay Michtom [email protected] (818) 368-1957 Jill Michtom [email protected] (818) 368-1957 Beginner’s Classes Calendar Jay Michtom [email protected] (818) 368-1957 On the Scene Jill Michtom [email protected] (818) 368-1957 Club Time Contact Location Club Directory Steve Himel [email protected] (949) 646-7082 CABRILLO INT'L FOLK Tue 7:00-8:00 (858) 459-1336 Georgina SAN DIEGO, Balboa Park Club Contributing Editor Richard Duree [email protected] (714) 641-7450 DANCERS Thu -

05 FB Guide.Qxp
Team Information 2005 Cardinal 2005 Stanford Football Player Summary Returning Defense (22) Lettermen Lost Starters Returning Starters Lost Lettermen *** Jon Alston, OLB (11; 4 offense, 7 defense) (15; 10 offense, 5 defense (7; 1 offense, 6 defense) (50; 25 offense, 22 defense, ** Calvin Armstrong, CB Offense (4) plus 2 kickers) Offense (1) * Emmanuel Awofadeju, OLB plus 3 specialists) *** Greg Camarillo, FL Offense (10) Alex Smith, TE * Bryan Bentrott, SS * Ryan Eklund, QB *** Casey Carroll, DT Mark Bradford, WR Defense (6) **** Alex Smith, TE Jon Cochran, LT Offense (25) *** Michael Craven, OLB **** Kenneth Tolon, RB Oshiomogho Atogwe, FS * Kris Bonifas, FB ** Taualai Fonoti, DE Ismail Simpson, LG David Bergeron, MLB ** Mark Bradford, WR ** Nick Frank, NT Defense (7) Brian Head, C Jared Newberry, OLB * Mikal Brewer, C ** Brandon Harrison, SS **** Oshiomogho Atogwe, FS Josiah Vinson, RG Scott Scharff, DE * Preston Clover, OG ** Trevor Hooper, SS *** David Bergeron, ILB Jeff Edwards, RT Leigh Torrence, CB ** Jon Cochran, OT *** Julian Jenkins, DE **** Jared Newberry, OLB Trent Edwards, QB Stanley Wilson, CB * Gerald Commissiong, RB * Landon Johnson, ILB *** Scott Scharff, DT J.R. Lemon, RB *** Gerren Crochet, FL * David Lofton, SS *** Will Svitek, DE Kris Bonifas, FB ** Patrick Danahy, TE * Matt McClernan, NT **** Leigh Torrence, CB Evan Moore, FL ** Jeff Edwards, OG ** Michael Okwo, OLB *** Stanley Wilson, CB Defense (5) ** Trent Edwards, QB *** Babatunde Oshinowo, NT Jon Alston, OLB *** Brian Head, C *** T.J. Rushing, CB Julian Jenkins, DT * Michael Horgan, TE * Nick Sanchez, CB Babatunde Oshinowo, NT * Ray Jones, RB *** Kevin Schimmelmann, OLB Kevin Schimmelmann, OLB *** J.R. Lemon, RB ** Mike Silva, ILB Brandon Harrison, SS * David Long, OT * Udeme Udofia, OLB ** David Marrero, RB ** Timi Wusu, OLB Kicker (2) *** Kyle Matter, QB Jay Ottovegio, P * Tim Mattran, OT Kickers / Specialists (3) Michael Sgroi, PK ** Justin McCullum, FL * Brent Newhouse, LS * Marcus McCutcheon, WR *Jay Ottovegio,P ** Evan Moore, WR *** Michael Sgroi, PK * T.C. -

Examensarbete
EXAMENSARBETE Att skydda kulturhistoriskt värdefulla byggnader mot brand Maria Ovesson Civilingenjörsexamen Brandteknik Luleå tekniska universitet Institutionen för samhällsbyggnad och naturresurser Att skydda kulturhistoriskt värdefulla byggnader mot brand Maria Ovesson Luleå tekniska universitet Brandingenjörsprogrammet Institutionen för samhällsbyggnad Maria Ovesson Att skydda kulturhistoriskt värdefulla byggnader mot brand Brandingenjörsprogrammet Institutionen för samhällsbyggnad Luleå tekniska universitet 971 87 Luleå Nomenklatur och förkortningar Nomenklatur avgiven värmeeffekt, HRR [kW] 2 HRR, 180 s medelvärde, konkalorimeter [kW/m ] 2 maximala HRR, konkalorimeter [kW/m ] α tillväxtfaktor [kW/s2] tig tid till antändning [s] Förkortningar BBR Boverkets byggregler BÄR Boverkets ändringsråd CFD Computational Fluid Dynamics EES Europeiska ekonomiska samarbetsområdet FDS Fire Dynamics Simulator FIGRA Fire Growth Rate, parameter för brandutvecklingshastighet HRR Heat release rate, värmeutveckling ISO International Organization for Standardization MSB Myndigheten för samhällsskydd och beredskap NIST National Institute of Standards and Technology RCT Room Corner Test SMOGRA Smoke Growth Rate, parameter för rökutvecklingshastighet SBI Single Burning Item test SP SP Sveriges Tekniska Forskningsinstitut THR600s Total värmeutveckling de första 600 sekunderna i SBI i Förord Förord Detta arbete utgör mitt examensarbete på 30 poäng och avslutar därmed min utbildning till civilingenjör i brandteknik på Luleå tekniska universitet. Arbetet har -

Emigrants from Gotland to America 1819-1890 Nils William Olsson
Swedish American Genealogist Volume 2 | Number 3 Article 3 9-1-1982 Emigrants from Gotland to America 1819-1890 Nils William Olsson Follow this and additional works at: https://digitalcommons.augustana.edu/swensonsag Part of the Genealogy Commons, and the Scandinavian Studies Commons Recommended Citation Olsson, Nils William (1982) "Emigrants from Gotland to America 1819-1890," Swedish American Genealogist: Vol. 2 : No. 3 , Article 3. Available at: https://digitalcommons.augustana.edu/swensonsag/vol2/iss3/3 This Article is brought to you for free and open access by Augustana Digital Commons. It has been accepted for inclusion in Swedish American Genealogist by an authorized editor of Augustana Digital Commons. For more information, please contact [email protected]. Emigrants from Gotland to America 1819-1890 Nils William Olsson The Provincial Archives (Landsarkivet) of the city of Vis by on the Swedish island of Gotland is the repository for the archival holdings of the island, includ ing not only the ecclesiastical records from the various parishes on the island, but also all of the records of the various official bodies, who for years have been administering the affairs of this, "the pearl of the Baltic." Among the many records housed in the Archives are those of the central administrative offices for the island, Got/ands Landskansli. In this vast body of material there is also a section dealing with the issuance of official passports for foreign travel - one of the duties of the central adminis tration. Until the early 1850's it was incumbent upon every Swedish citizen as well as aliens, to procure a passport before departing for foreign parts. -

Stanford Football
2005 Stanford Football Welcome to Stanford Football • Tradition of Excellence • Competitive Pacific-10 Conference and Non-Conference Schedule • Famous Rivalries • National Television Exposure • All-America Selections • NFL Draft Picks • Bowl Games • National Honors and Awards • Gameday at Stanford Stadium • Outstanding Athletic Facilities • The Most Successful Collegiate Athletic Program in the United States • World Renowned Academics • Perfect Weather All Year Long • A Beautiful Campus in One of the Country’s Most Desirable Regions 2005 STANFORD FOOTBALL 1 2005 Stanford Football The Stanford- NFL Connection Stanford has produced Super Bowl Champions, Super Bowl MVPs, Hall of Fame players and coaches, and John Lynch numerous NFL greats. Denver Broncos Over 30 former Cardinal players began the 2005 season on NFL rosters. Stanford has had 13 players selected in Stanford in the NFL the last three NFL Drafts, and 26 in the last seven years, among the most in the nation. Tank Williams Some of Stanford’s NFL players and Tennessee Titans coaches include: • Brian Billick, coach • John Brodie • John Elway – NFL Hall of Fame • Darrien Gordon • Dennis Green, coach • Kwame Harris • James Lofton – NFL Hall of Fame • John Lynch • Ed McCaffrey • Ernie Nevers – NFL Hall of Fame Eric Heitmann • Darrin Nelson San Francisco 49ers • Ken Margerum • Jim Plunkett • Jon Ritchie • George Seifert, coach • Dick Vermeil, coach • Troy Walters • Bill Walsh, coach – NFL Hall of Fame • Gene Washington • Bob Whitfield • Tank Williams • Kailee Wong Coy Wire Buffalo Bills -

The Cultural Heritage of the Swedish Immigrant: Selected Refer- Ences
Digitized by the Internet Archive in 2011 with funding from University of Illinois Urbana-Champaign http://www.archive.org/details/culturalheritageOOande AUGUSTANA LIBRARY PUBLICATIONS Number 27 LUCIEN WHITE, General Editor / h The CULTURAL HERITAGE of the SWEDISH IMMIGRANT Selected Rererences By O. FRITIOF ANDER ROCK ISLAND, ILLINOIS AUGUSTANA COLLEGE LIBRARY 1956 AUGUSTANA LIBRARY PUBLICATIONS 1. The Mechanical Composition of Wind Deposits. By Johan August Udden (1898) $1.00 2. An Old Indian Village. By Johan August Udden (1900) 1.00 3. The Idyl in German Literature. By Gustav Andreen (1902) 1.00 4. On the Cyclonic Distribution of Rainfall. Bv Johan August Udden (1905) io: 5. Fossil Mastodon and Mammoth Remains in Illinois and Iowa. By Netta C. Anderson. Proboscidian Fossi.s of the Pleistocene Depos- its in Illinois and Iowa. By Johan August Udden (1905) 1.00 6. Scandinavians Who Have Contributed to the Knowledge of the Flora of North America. By Per Axel Rydberg. A Geological Survey of Lands Belonging to the New York and Texas Land Company, Ltd., in the Upper Rio Grande Embayment in Texas. By John August Udden (1907) O. P. 7. Genesis and Development of Sand Formations on Marine Coasts. By Pehr Olsson-Seffer. The Sand Strand Flora of Marine Coasts By Pehr Olsson-Seffer (1910) IjOO 8. Alternative Readings in the Hebrew of the Books of Samuel. By Otto H. Bostrom (1918) 11 9. On the Solution of the Differential Equations of Motion of a Dou- ble Pendulum. By William E. Cederberg (1923) 75 10. The Danegeld in France. By Einar Joranson (1924) 1.25 11. -

The Arnold Strongman Classic
THE JOURNAL OF PHYSICAL CULTURE Volume 9 Number 1August 2005 The Arnold Strongman Classic In early March of 2005 the fourth annual contest in 2002, but in 2003 and 2004, the contest has Strongman Classic was held in Columbus, Ohio as one been dominated by Zydrunas Savickas, the powerful of the fifteen sporting events comprising the gigantic young Lithuanian giant who has gotten stronger each sports festival now known as year. Broad, tall, and athletic, the Arnold Fitness Weekend. the 6'3", 375 pound Savickas The 2002 and 2003 versions is ideally constructed for featured four events, the 2004 events which require a com- version featured five events, bination of brute strength and and the 2005 version added a power. We have done our sixth event. The 2005 com- best to limit the importance petition took two days, of endurance in our competi- involved ten athletes, and fea- tions, as our intention has tured three events on each of been, and remains, to deter- those two days. The aim of mine which athlete has the this annual competition, from best claim to the mythical the beginning, has been to title of "The Strongest Man in design a series of events the World." We realize, of which—taken together—pro- course, that the winner of our vide a way to determine a contest—or any other such man's overall strength. contest, for that matter—will Those of us responsible for face counter-claims from oth- the choice of events—David er men and other contests, but Webster, Bill Kazmaier, Jan we nonetheless accepted the Todd, and myself—have challenge given to us five done our best to create events years ago by Arnold that would allow the top per- Schwarzenegger and Jim formers in weightlifting, Lorimer to put together a powerlifting, and the "strong- contest and a group of man" sport to have an equal Referee Odd Haugen calls the count as Lithuania's strength athletes that would Zydrunas Savickas lifted the Inch Dumbell overhead chance of winning. -

Swedish American Genealogist
Swedish American Genealogist Volume 16 Number 4 Article 1 12-1-1996 Full Issue Vol. 16 No. 4 Follow this and additional works at: https://digitalcommons.augustana.edu/swensonsag Part of the Genealogy Commons, and the Scandinavian Studies Commons Recommended Citation (1996) "Full Issue Vol. 16 No. 4," Swedish American Genealogist: Vol. 16 : No. 4 , Article 1. Available at: https://digitalcommons.augustana.edu/swensonsag/vol16/iss4/1 This Full Issue is brought to you for free and open access by the Swenson Swedish Immigration Research Center at Augustana Digital Commons. It has been accepted for inclusion in Swedish American Genealogist by an authorized editor of Augustana Digital Commons. For more information, please contact [email protected]. (ISSN 0275-9314) Swedish American Genealo ist A journal devoted to Swedish American biography, genealogy and personal history CONTENTS Swedish Parish Records in North America by Lars-Goran Johansson 257 Hulda Johannesson from Jonkoping by Lila and Wendy Kirkwood 2 7 4 Book Reviews by Ronald J. Johnson and Nils William Olsson 2 7 9 Genealogical Queries 2 8 2 Index of Personal Names 292 Index of Place Names 3 O 7 Vol. XVI December 1996 No. 4 Swedish America n \ ·Genealogist�� \ Copynght © 1996 (ISSN 0275- 93l4) \ Swedish American Genealogist Swenson Swedish Immigration Research Center Augustana College Rock Island, IL 61201 Tel. (309J 794 7204 Publisher: Swenson Swedish Immigration Research Center Editor: Nils William Olsson, Ph.D., F.A.S.G., P.O. Box 2186, Winter Park, FL 32790. Tel. (407) 647 4292 \ Associate Editor, James E. Erick�on, Ph.D.,Edina. MN Contributing Editor, Peter Stebbins Craig, J.D., F.A.S.G., Washington, DC l Editorial Committee: Dag Blanck, Uppsala, Sweden Glen E. -

Mediakit Contents
01 Mediakit CONTENTS page 03 › GENERAL SYNOPSIS page 04 › THE EPISODES page 14 › THE CHARACTERS page 21 › THE PRODUCTION page 24 › CONTACT page 02 For further information, please contact ZDFE.drama P + 49 (0) 6131–991 1855 | F + 49 (0) 6131–991 2855 [email protected] | www.zdf-enterprises.de GENERAL SYNOPSIS Arne Dahl’s A Unit has been disbanded for the last two years. When a wave of brutal murders hits Polish nurses in Sweden, the National Police see their chance to instate the unit again. Kerstin Holm, previously a member of the A Unit, is assigned to lead them. The A Units official role in the police force is to investigate complex, violent crimes with international connections. Kerstin builds her team with former members of the unit Nyberg, Chavez, Svenhagen and Söderstedt, but she is assigned a new recruit as well, somewhat reluctantly – Ida Jankowicz, a wiry, yet powerful, rookie with exceptional language skills. Paul Hjelm, previously a key figure in the A Unit, has been promoted to a job as head of Internal Affairs. At first, he and the A Unit are pretty far removed from each other, but their paths will cross sooner than any of them could imagine. We meet a chastened unit of individuals who have allowed the all consuming nature of their police work to eat away at their private lives. Demands and expectations have never been higher and a cold wind blows through the corridors at the National Police headquarters. Can Kerstin get the unit to deliver or is this new effort a misguided attempt by a paranoid police force in a time of increasingly unusual and refined criminal activity? page 03 For further information, please contact ZDFE.drama P + 49 (0) 6131–991 1855 | F + 49 (0) 6131–991 2855 [email protected] | www.zdf-enterprises.de EP. -

Sunniva Abelli Masterarbete 2016
Kurs: AG1015 Självständigt arbete 30 hp 2016 Konstnärlig masterexamen i musik Institutionen för folkmusik Handledare: Olof Misgeld Sunniva Abelli How to become a musical super-tool? To play, dance and sing with the nyckelharpa Skriftlig reflektion inom självständigt arbete Inspelning av det självständiga, konstnärliga arbetet finns dokumenterat i det tryckta exemplaret av denna text på KMH:s bibliotek. I Contents Preface ........................................................................................................................ 1 My#artistic#background# Acknowledgements# 1. Introduction, purpose, idea ..................................................................................... 2 1.1#What#is#a#'Musical#Super=Tool'?## 1.2#My#questions# 2. Background............................................................................................................. 3 2.1#Background# 2.2#Multitasking# 2.3#Ki=ergonomics#–#body#as#the#main#instrument# 2.4#Burträskpolkett#and#other#Nordic#polka#styles,#to#play#for#dancing# 2.5#Flamenco#and#other#foot#percussion#techniques# 2.6#Expression,#Ngoma#–#holistic#art# # 3. Material and method ............................................................................................... 7 3.1#Analysing#polka#(Burträskpolkett)#=#the#connection#between#dance#and# music# 3.2#Foot#percussion#technique# 3.3#Playfulness#and#courage#as#a#method:#Improvisation#and#developing#my# voice# 3.4#Learning#karelian#music#and#dance## 3.5#Ideas#along#the#way,#the#development# 4. Result ...................................................................................................................