Chironomid-Based Inference Models for Estimating Mean July Air Temperature and Water Depth from Lakes in Yakutia, Northeastern Russia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Download The
AN ECOLOGICAL STUDY OF SOME OF THE CHIRONOMIDAE INHABITING A SERIES OF SALINE LAKES IN CENTRAL BRITISH COLUMBIA WITH SPECIAL REFERENCE TO CHIRONOMUS TENTANS FABRICIUS by Robert Alexander Cannings BSc. Hons., University of British Columbia, 1970 A THESIS SUBMITTED IN PARTIAL FULFILMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE in the Department of Zoology We accept this thesis as conforming to the required standard THE UNIVERSITY OF BRITISH COLUMBIA May, 1973 In presenting this thesis in partial fulfilment of the requirements for an advanced degree at the University of British Columbia, I agree that the Library shall make it freely available for reference and study. I further agree that permission for extensive copying of this thesis for scholarly purposes may be granted by the Head of my Department or by his representatives. It is understood that copying or publication of this thesis for financial gain shall not be allowed without my written permission. Department of The University of British Columbia Vancouver 8, Canada Date ii ABSTRACT This thesis is concerned with a study of the Chironomidae occuring in a saline lake series in central British Columbia. It describes the ecological distribution of species, their abundance, phenology and interaction, with particular attention being paid to Chironomus tentans. Emphasis is placed on the species of Chironomus that coexist in these lakes and a further analysis is made of the chromo• some inversion frequencies in C. tentans. Of the thirty-four species represented by identifiable adults in the study, eleven species have not been previously reported in British Columbia, five are new records for Canada and seven species are new to science. -

Makrozoobentos Kao Pokazatelj Ekološkog Potencijala Umjetnih Stajaćica
Makrozoobentos kao pokazatelj ekološkog potencijala umjetnih stajaćica Vučković, Natalija Doctoral thesis / Disertacija 2021 Degree Grantor / Ustanova koja je dodijelila akademski / stručni stupanj: University of Zagreb, Faculty of Science / Sveučilište u Zagrebu, Prirodoslovno-matematički fakultet Permanent link / Trajna poveznica: https://urn.nsk.hr/urn:nbn:hr:217:251464 Rights / Prava: In copyright Download date / Datum preuzimanja: 2021-10-11 Repository / Repozitorij: Repository of Faculty of Science - University of Zagreb PRIRODOSLOVNO-MATEMATIČKI FAKULTET BIOLOŠKI ODSJEK Natalija Vučković MAKROZOOBENTOS KAO POKAZATELJ EKOLOŠKOG POTENCIJALA UMJETNIH STAJAĆICA DOKTORSKI RAD Zagreb, 2020 PRIRODOSLOVNO-MATEMATIČKI FAKULTET BIOLOŠKI ODSJEK Natalija Vučković MAKROZOOBENTOS KAO POKAZATELJ EKOLOŠKOG POTENCIJALA UMJETNIH STAJAĆICA DOKTORSKI RAD Mentor: Prof. dr. sc. Zlatko Mihaljević Zagreb, 2020 FACULTY OF SCIENCE DIVISION OF BIOLOGY Natalija Vučković MACROZOOBENTHOS AS AN INDICATOR OF THE ECOLOGICAL POTENTIAL OF CONSTRUCTED LAKE DOCTORAL DISSERTATION Supervisor: Prof. dr. sc. Zlatko Mihaljević Zagreb, 2020 Ovaj je doktorski rad izrađen na Zoologijskom zavodu Prirodoslovno- matematičkog fakulteta, pod vodstvom Prof. dr. sc. Zlatka Mihaljevića, u sklopu Sveučilišnog poslijediplomskog doktorskog studija Biologije pri Biološkom odsjeku Prirodoslovno-matematičkog fakulteta Sveučilišta u Zagrebu. MENTOR DOKTORSKE DISERTACIJE Prof. dr. sc. Zlatko Mihaljević Rođen je 21. siječnja 1966. godine u Varaždinu. Studij biologije (ekologija), upisuje 1986. -

DNA Barcoding
Full-time PhD studies of Ecology and Environmental Protection Piotr Gadawski Species diversity and origin of non-biting midges (Chironomidae) from a geologically young lake PhD Thesis and its old spring system Performed in Department of Invertebrate Zoology and Hydrobiology in Institute of Ecology and Environmental Protection Różnorodność gatunkowa i pochodzenie fauny Supervisor: ochotkowatych (Chironomidae) z geologicznie Prof. dr hab. Michał Grabowski młodego jeziora i starego systemu źródlisk Auxiliary supervisor: Dr. Matteo Montagna, Assoc. Prof. Łódź, 2020 Łódź, 2020 Table of contents Acknowledgements ..........................................................................................................3 Summary ...........................................................................................................................4 General introduction .........................................................................................................6 Skadar Lake ...................................................................................................................7 Chironomidae ..............................................................................................................10 Species concept and integrative taxonomy .................................................................12 DNA barcoding ...........................................................................................................14 Chapter I. First insight into the diversity and ecology of non-biting midges (Diptera: Chironomidae) -

CHIRONOMUS NEWSLETTER on CHIRONOMIDAE RESEARCH Co-Editors: Ruth CONTRERAS-LICHTENBERG Naturhistorisches Museum Wien, Burgring 7, A-1014 WIEN, Austria Peter H
CHIRONOMUS NEWSLETTER ON CHIRONOMIDAE RESEARCH Co-Editors: Ruth CONTRERAS-LICHTENBERG Naturhistorisches Museum Wien, Burgring 7, A-1014 WIEN, Austria Peter H. LANGTON 5 Kylebeg Avenue, Mountsandel, Coleraine, Co. Londonderry, Northern Ireland, BT52 1JN - Northern Ireland Bibliography: Odwin HOFFRICHTER Institut f. Biologie I, Albert-Ludwigs-Universität Freiburg, Hauptstrasse 1 D-79104 , Germany Treasurer: Trond ANDERSEN: Museum of Zoology, University of Bergen, Museplass 3, N-5007 Bergen - Norway ISSN 0172-1941 No. 13 September 2000 CONTENTS Chironomid Work in Munich to Continue ............................................................................................................... 1 New curator at the Zoologische Staatssammlung Munich ...................................................................................... 2 Contributions in SPIXIANA in Memory of Dr. Reiss.............................................................................................. 4 To Iya Kiknadze at 70................................................................................................................................................ 5 Current Research ....................................................................................................................................................... 7 Short – Communications ......................................................................................................................................... 19 Notice Board ................................................................................................................................... -
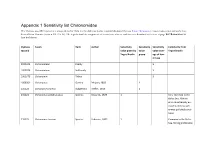
Appendix 1 Sensitivity List Chironomidae the Dyntaxa Taxonid Represents a Unique Identifier from the Swedish Taxonomic Standard Database Dyntaxa (
Appendix 1 Sensitivity list Chironomidae The Dyntaxa taxonID represents a unique identifier from the Swedish taxonomic standard database Dyntaxa (http://dyntaxa.se). Taxon names rank and author are derived from Dyntaxa (version 2013-06-26). The logic behind the assignment of a sensitivity class to each taxon is described in the text at page Fel! Bokmärket är inte definierat.. Dyntaxa Taxon Rank Author Sensitivity Sensitivity Sensitivity Comments from taxonid value given by value value aver- Yngve Brodin Yngve Brodin group age of low- er taxa 2001302 Chironomidae Family 5 1009974 Chironominae Subfamily 5 1009975 Chironomini Tribus 5 1009301 Chironomus Genera Meigen, 1803 1 235223 Camptochironomus Subgenera Kieffer, 1918 1 235224 Chironomus pallidivittatus Species Edwards, 1929 1 Very common in the Baltic Sea. Able to endure extremely eu- trophic and also oth- erwise polluted condi- tions. 235225 Chironomus tentans Species Fabricius, 1805 1 Common in the Baltic Sea. Strong preference WATERS: A PROBABILITY BASED INDEX FOR BENTHIC ASSESSMENT IN THE BALTIC SEA Dyntaxa Taxon Rank Author Sensitivity Sensitivity Sensitivity Comments from taxonid value given by value value aver- Yngve Brodin Yngve Brodin group age of low- er taxa for eutrophic and even extremely eutrophic and polluted condi- tions. 235228 Chironomus Subgenera Meigen, 1803 1 235234 Chironomus annularius Species Meigen, 1818 1 Rather common in the Baltic Sea, easily con- fused with several other Chironomus species bout as larvae and adults. 235235 Chironomus anthracinus Species Zetterstedt, 1860 1 Common north of Åland, otherwise less common in the Baltic Sea. Mainly found below the littoral. Prefers less strongly 2 WATERS: A PROBABILITY BASED INDEX FOR BENTHIC ASSESSMENT IN THE BALTIC SEA Dyntaxa Taxon Rank Author Sensitivity Sensitivity Sensitivity Comments from taxonid value given by value value aver- Yngve Brodin Yngve Brodin group age of low- er taxa eutrophic conditions than C. -

Carew Cait 2020 Msc.Pdf (3.317Mb)
CHIRONOMID AUTOECOLOGY OF THE PAST AND PRESENT, AND A CAUSAL ANALYSIS OF RECREATIONAL SHORELINE DEVELOPMENTS ON HYPOLIMNETIC OXYGEN IN ALGONQUIN PARK LAKES CAIT CAREW A THESIS SUBMITTED TO THE FACULTY OF GRADUATE STUDIES IN PARTIAL FULFILMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE GRADUATE PROGRAM IN BIOLOGY, YORK UNIVERSITY, TORONTO, ONTARIO MARCH 2020 © CAIT CAREW, 2020 Abstract Recreational cottages continue to be leased within Algonquin Park despite inadequate assessments of cottage impacts on lake water quality and ecosystem integrity. Cottages can increase phosphorus export to lakes, resulting in increased productivity and declines in hypolimnetic oxygen. Algonquin Park lakes contain dense populations of lake trout (Salvelinus namaycush) and brook trout (Salvelinus fontinalis), which are sensitive to declining hypolimnetic oxygen. Dipteran subfossil remains were used to calibrate a VWHO inference model (RMSEP = -1 1.7 mg O2 L ) to determine baseline VWHO (historical, pre-European settlement, < ca. 1850 CE) and assess VWHO change since then using a top-bottom paleolimnological approach. Despite increased anthropogenic activity in the park, inferred VWHO did not change predictably since circa 1850. We did not detect a significant effect of cottages on VWHO. However, regional declines in phosphorus export may be responsible for muting the effects of anthropogenic phosphorus inputs on VWHO in Algonquin Park lakes. ii Acknowledgements Here, acknowledge those who assisted me, in some way, along my path to completing this thesis. First, I would like to thank my supervisor, Roberto Quinlan, you have been an incredible support since we met many years ago. I am grateful to have had this opportunity to learn from such a kind and ‘always’ funny individual. -

(Insecta: Diptera) in Lowland Running Waters of North-East Germany (Brandenburg) Based on 10- Year EU-Water Framework Directive Monitoring Programme
37 Lauterbornia 77: 37-62, D-86424 Dinkelscherben, 2014-07-07 Faunistic overview of Chironomidae (Insecta: Diptera) in lowland running waters of north-east Germany (Brandenburg) based on 10- year EU-Water Framework Directive monitoring programme Claus Orendt, Xavier-François Garcia, Berthold F. Janecek, Susanne Michiels, Claus-Joachim Otto and Reinhard Müller With 8 figures and 5 tables Keywords : Chironomidae, Diptera, Insecta, Brandenburg, Germany, Central European Lowlands, running water, check list, faunistics, larva, pupa, pupal exuviae, imago Schlagwörter : Chironomidae, Diptera, Insecta, Brandenburg, Deutschland, Zentrales Flachland, Fließgewässer, Checkliste, Faunistik, Larve, Puppe, Imago The results of a 10-year monitoring programme are used to provide a list of the Chironomidae taxa of running waters in Brandenburg, north-east Germany, Central European Lowlands ecoregion. The 573 taxa recorded, represent 58 % of the Chironomidae in the "Taxa list of the freshwater organisms of Germany". The 408 re- cords with a valid species status include 56 % of the species, and the 121 genera include 73 % of the genera of the German Chironomidae fauna. The records are based on collections of all developmental stages and were de- rived from about 2350 samples taken during a 10-year monitoring programme up to 2013. The study shows how monitoring programmes with a clear strategy and proper data storage and management can make a sub- stantial contribution to knowledge of the fauna of an ecoregion and provide a solid database for ecological ana- lyses. Further data evaluation indicated an affinity of certain taxa to a particular water type, but further in- depth analysis is required. 1 Introduction Until the end of the last century understanding about the distribution of Chironomidae in the northern lowlands of Germany was limited, because they were seldom included in waterbody studies. -

The Larval Chironomidae (Diptera) Fauna of Gökçeada (Imbroz)
G.U. Journal of Science 19(2): 69-75 (2006) www.gujs.org The Larval Chironomidae (Diptera) Fauna of Gökçeada (Imbroz) Nurcan ÖZKAN♣ Trakya University, Education Faculty, Department of Primary Education, 22030, Edirne, TURKEY Received: 04. 10. 2004 Accepted: 16. 09. 2005 ABSTRACT Benthos specimens were collected by hand mud scoop from 21 localities in Gökçeada during each field work, between 06-28.07.1991 and 06-10.08.1999. Specimens were collected from every kind of habitat as possible (e.g. lakes, ponds, dams, streams and troughs). As a result of this study 53 species of 34 genuses were determined from subfamilies Tanypodinae, Orthocladiinae and Chironominae (Chironomini and Tanytarsini) of Chironomidae family. 35 of the 53 species that were determined are new records for the larval Chironomidae fauna of Gökçeada. 25, the species number described in previous studies in Gökçeada, has increased to 60 with this study. Key Words: Diptera, Chironomidae, Limnofauna, Gökçeada (Imbroz), TURKEY. 1. INTRODUCTION 1.1 Description of Work Field 2. MATERIAL and METHOD Gökçeada is in the North east region of the Aegean Benthos specimens were collected with hand mud scoop sea which occurred by the breaking and collapsement of from 21 localities in Gökçeada during each field work, the ground at the end of the 3rd and at the beginning of between 06-28.07.1991 and 06-10.08.1999. They were the 6th geological era, approximately 2-2,5 million years fixed with 70% alcohol and brought to the laboratory ago, and it is the continuity of Anatolia and Thrace [1, 2, (Figure 1). -

An Updated List of Chironomid Species from Italy with Biogeographic Considerations (Diptera, Chironomidae)
Biogeographia – The Journal of Integrative Biogeography 34 (2019): 59–85 An updated list of chironomid species from Italy with biogeographic considerations (Diptera, Chironomidae) BRUNO ROSSARO1, NICCOLÒ PIROLA1, LAURA MARZIALI2, GIULIA MAGOGA1, ANGELA BOGGERO3, MATTEO MONTAGNA1 1 Dipartimento di Scienze Agrarie e Ambientali (DiSAA), University of Milano, Via Celoria 2, 20133 Milano (Italy) 2 Water Research Institute - National Research Council (IRSA-CNR), Via del Mulino 19, 20861 Brugherio (MB) (Italy) 3 Water Research Institute - National Research Council (IRSA-CNR), Corso Tonolli 50, 28922 Verbania Pallanza (Italy) * corresponding author: [email protected] Keywords: biodiversity, checklist, faunistics, freshwaters, non-biting midges, species list. SUMMARY In a first list of chironomid species from Italy from 1988, 359 species were recognized. The subfamilies represented were Tanypodinae, Diamesinae, Prodiamesinae, Orthocladiinae and Chironominae. Most of the species were cited as widely distributed in the Palearctic region with few Mediterranean (6), Afrotropical (19) or Panpaleotropical (3) species. The list also included five species previously considered Nearctic. An updated list was thereafter prepared and the number of species raised to 391. Species new to science were added in the following years further raising the number of known species. The list of species known to occur in Italy is now updated to 580, and supported by voucher specimens. Most species have a Palearctic distribution, but many species are distributed in other biogeographical regions; 366 species are in common with the East Palaearctic region, 281 with the Near East, 248 with North Africa, 213 with the Nearctic, 104 with the Oriental, 23 species with the Neotropical, 23 with the Afrotropical, 16 with the Australian region, and 46 species at present are known to occur only in Italy. -

Chironomid Community Dynamics in Enol Lake (Picos De Europa
P. Tarrats, M. Rieradevall, N. Prat, M.P. Mata, M. Morellón, J. Vegas, J. Sánchez, B.L. Valero, A. Moreno Geneva 5-10 July 2015 : Global Change in Mountain Lakes Study and understand the present to explain the past “Paleoecological reconstructions using biological registers need a good knowledge of the actual ecology and autoecology of the species” (Birks and Birks, 2004). “It is important to combine paleoecological data with those obtained from instrumental series and collected from ecological techniques used in the study of actual ecosystems conditions” (Peng et al., 2011) CHIRONOMIDAE What? . Insecta:Diptera . Aquatics and semi-aquatic . Larvae inhabit lake’s benthos . In paleo: larvae head capsules Why? . High taxonomic diversity and abundances . The presence of different species reflects different ecological conditions . Taxonomy (identification): . Preservation (Paleo): Chironomid life cycle and larvae We aim to study and understand the actual Chironomidae community in order to improve the interpretation of the chironomid subfossil record To characterize the modern chironomid community of Enol Lake. To evaluate which factors are responsible of changes in community: . Abundance . Composition Enol Lake Location North Spain (Asturias) Picos de Europa National Park Origin Glacial Altitude 1070 m asl Max depth 22 m Surface Area 12.2 ha Location map of Enol Lake Enol Lake T (ºC) DO (%) 5 10 15 20 25 0 25 50 75 100 125 150 Lake type Warm monomictic 0 0 Trophic state Oligotrophic 2 2 4 4 6 6 Lake water chemistry 8 8 Hard water HCO - & Ca ) (m) 3 10 (m 10 12 12 Depth Alkaline Depth 2- 2- 14 14 Low nutrient NO3 , NO 16 May 16 contents NH4+ &PO 3- May 4 July 18 18 July September 20 20 September November November 22 22 No depth patterns Temperature and DO profiles of 4 samplings (May, July, September and November) of 2013 in Enol Lake. -

Diptera Families MS FINAL
The Diptera Families of British Columbia The Diptera Families of British Columbia G.G.E. Scudder and R.A. Cannings March 31, 2006 G.G.E. Scudder and R.A. Cannings Printed 04/25/06 Coleoptera Families of British Columbia Table of Contents Introduction......................................................................................................................................1 Order Diptera Description................................................................................................................3 Keys to Order Diptera and Families.................................................................................................6 Family Descriptions .......................................................................................................................26 Suborder NEMATOCERA............................................................................................................26 Infraorder TIPULOMORPHA .......................................................................................................26 Family TANYDERIDAE (Primitive Crane Flies) [Fig. 1]............................................................26 Family TIPULIDAE (Crane Flies) [Fig. 2]....................................................................................26 Infraorder BLEPHARICEROMORPHA .......................................................................................27 Family BLEPHARICERIDAE (Net-winged Midges) [Fig. 3]......................................................27 Family DEUTEROPHLEBIIDAE (Mountain -

Key Factors for Biodiversity of Urban Water Systems
Key factors for biodiversity of urban water systems Kim Vermonden Key factors for biodiversity of urban water systems Vermonden, K., 2010. Key factors for biodiversity of urban water systems. PhD-thesis, Radboud University, Nijmegen. © 2010 K. Vermonden, all rights reserved. ISBN: 978-94-91066-01-6 Layout: A. M. Antheunisse Printed by: Ipskamp Drukkers BV, Enschede This project was financially supported by the Interreg IIIb North-West Europe programme Urban water and the municipalities of Nijmegen and Arnhem. Key factors for biodiversity of urban water systems Een wetenschappelijke proeve op het gebied van de Natuurwetenschappen, Wiskunde en Informatica PROEFSCHRIFT ter verkrijging van de graad van doctor aan de Radboud Universiteit Nijmegen op gezag van de rector magnificus prof. mr. S.C.J.J. Kortmann, volgens besluit van het college van decanen in het openbaar te verdedigen op donderdag 25 november 2010 om 10.30 uur precies door Kim Vermonden geboren op 20 november 1980 te Breda Promotores: Prof. dr. ir. A.J. Hendriks Prof. dr. J.G.M. Roelofs Copromotores: Dr. R.S.E.W. Leuven Dr. G. van der Velde Manuscriptcommissie: Prof. dr. H. Siepel (voorzitter) Prof. dr. A.J.M. Smits Dr. J. Borum (Kopenhagen Universiteit, Denemarken) Contents Chapter 1 Introduction 9 Chapter 2 Does upward seepage of river water and storm water runoff 19 determine water quality of urban drainage systems in lowland areas? A case study for the Rhine-Meuse delta (Hydrological Processes 23: 3110-3120) Chapter 3 Species pool versus site limitations of macrophytes in urban 39 waters (Aquatic Sciences 72: 379-389) Chapter 4 Urban drainage systems: An undervalued habitat for aquatic 59 macroinvertebrates (Biological Conservation 142: 1105-1115) Chapter 5 Key factors for chironomid diversity in urban waters 81 (submitted) Chapter 6 Environmental factors determining invasibility of urban 103 waters for exotic macroinvertebrates (submitted to Diversity and Distributions) Chapter 7 Synthesis 121 Summary 133 Samenvatting 137 Dankwoord 141 Curriculum vitae 145 Urban water system Nijmegen.