Carew Cait 2020 Msc.Pdf (3.317Mb)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Download The
AN ECOLOGICAL STUDY OF SOME OF THE CHIRONOMIDAE INHABITING A SERIES OF SALINE LAKES IN CENTRAL BRITISH COLUMBIA WITH SPECIAL REFERENCE TO CHIRONOMUS TENTANS FABRICIUS by Robert Alexander Cannings BSc. Hons., University of British Columbia, 1970 A THESIS SUBMITTED IN PARTIAL FULFILMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE in the Department of Zoology We accept this thesis as conforming to the required standard THE UNIVERSITY OF BRITISH COLUMBIA May, 1973 In presenting this thesis in partial fulfilment of the requirements for an advanced degree at the University of British Columbia, I agree that the Library shall make it freely available for reference and study. I further agree that permission for extensive copying of this thesis for scholarly purposes may be granted by the Head of my Department or by his representatives. It is understood that copying or publication of this thesis for financial gain shall not be allowed without my written permission. Department of The University of British Columbia Vancouver 8, Canada Date ii ABSTRACT This thesis is concerned with a study of the Chironomidae occuring in a saline lake series in central British Columbia. It describes the ecological distribution of species, their abundance, phenology and interaction, with particular attention being paid to Chironomus tentans. Emphasis is placed on the species of Chironomus that coexist in these lakes and a further analysis is made of the chromo• some inversion frequencies in C. tentans. Of the thirty-four species represented by identifiable adults in the study, eleven species have not been previously reported in British Columbia, five are new records for Canada and seven species are new to science. -

Growth and Development of Chironomus Thummi (Kieffer) on Various Algal Diets
University of Massachusetts Amherst ScholarWorks@UMass Amherst Masters Theses 1911 - February 2014 1979 Growth and development of Chironomus thummi (Kieffer) on various algal diets. Daniel Weissman University of Massachusetts Amherst Follow this and additional works at: https://scholarworks.umass.edu/theses Weissman, Daniel, "Growth and development of Chironomus thummi (Kieffer) on various algal diets." (1979). Masters Theses 1911 - February 2014. 3035. Retrieved from https://scholarworks.umass.edu/theses/3035 This thesis is brought to you for free and open access by ScholarWorks@UMass Amherst. It has been accepted for inclusion in Masters Theses 1911 - February 2014 by an authorized administrator of ScholarWorks@UMass Amherst. For more information, please contact [email protected]. GROWTH AND DEVELOPMENT OF CHIRONOMUS THUMMI (KIEFFER) ON VARIOUS ALGAL DIETS A Thesis Presented By Daniel Weissman Submitted to the Graduate School of the University of Massachusetts in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE V September 1979 Entomology GROWTH AND DEVELOPMENT OF CHIRONOMUS THUMMI (KIEFFER) ON VARIOUS ALGAL DIETS A Thesis Presented By Daniel Weissman Approved as to style and content by: John D. Edman, Ph.D., Chairman of Committee y lames B. Knng, .D ' /Department Head / Entomology n ACKNOWLEDGMENTS I thank my advisor, Dr. John Edman, and,Dr. James Kring, Department Head, for their positive attitude and continual encouragement during my two years of study and research. I am grateful to my committee members, Dr, Pedro Barbosa and Dr. T. Michael Peters, for directing my attention to important problems in my investigation. Thanks are also extended to: Dr. Robert R. -

Makrozoobentos Kao Pokazatelj Ekološkog Potencijala Umjetnih Stajaćica
Makrozoobentos kao pokazatelj ekološkog potencijala umjetnih stajaćica Vučković, Natalija Doctoral thesis / Disertacija 2021 Degree Grantor / Ustanova koja je dodijelila akademski / stručni stupanj: University of Zagreb, Faculty of Science / Sveučilište u Zagrebu, Prirodoslovno-matematički fakultet Permanent link / Trajna poveznica: https://urn.nsk.hr/urn:nbn:hr:217:251464 Rights / Prava: In copyright Download date / Datum preuzimanja: 2021-10-11 Repository / Repozitorij: Repository of Faculty of Science - University of Zagreb PRIRODOSLOVNO-MATEMATIČKI FAKULTET BIOLOŠKI ODSJEK Natalija Vučković MAKROZOOBENTOS KAO POKAZATELJ EKOLOŠKOG POTENCIJALA UMJETNIH STAJAĆICA DOKTORSKI RAD Zagreb, 2020 PRIRODOSLOVNO-MATEMATIČKI FAKULTET BIOLOŠKI ODSJEK Natalija Vučković MAKROZOOBENTOS KAO POKAZATELJ EKOLOŠKOG POTENCIJALA UMJETNIH STAJAĆICA DOKTORSKI RAD Mentor: Prof. dr. sc. Zlatko Mihaljević Zagreb, 2020 FACULTY OF SCIENCE DIVISION OF BIOLOGY Natalija Vučković MACROZOOBENTHOS AS AN INDICATOR OF THE ECOLOGICAL POTENTIAL OF CONSTRUCTED LAKE DOCTORAL DISSERTATION Supervisor: Prof. dr. sc. Zlatko Mihaljević Zagreb, 2020 Ovaj je doktorski rad izrađen na Zoologijskom zavodu Prirodoslovno- matematičkog fakulteta, pod vodstvom Prof. dr. sc. Zlatka Mihaljevića, u sklopu Sveučilišnog poslijediplomskog doktorskog studija Biologije pri Biološkom odsjeku Prirodoslovno-matematičkog fakulteta Sveučilišta u Zagrebu. MENTOR DOKTORSKE DISERTACIJE Prof. dr. sc. Zlatko Mihaljević Rođen je 21. siječnja 1966. godine u Varaždinu. Studij biologije (ekologija), upisuje 1986. -

DNA Barcoding
Full-time PhD studies of Ecology and Environmental Protection Piotr Gadawski Species diversity and origin of non-biting midges (Chironomidae) from a geologically young lake PhD Thesis and its old spring system Performed in Department of Invertebrate Zoology and Hydrobiology in Institute of Ecology and Environmental Protection Różnorodność gatunkowa i pochodzenie fauny Supervisor: ochotkowatych (Chironomidae) z geologicznie Prof. dr hab. Michał Grabowski młodego jeziora i starego systemu źródlisk Auxiliary supervisor: Dr. Matteo Montagna, Assoc. Prof. Łódź, 2020 Łódź, 2020 Table of contents Acknowledgements ..........................................................................................................3 Summary ...........................................................................................................................4 General introduction .........................................................................................................6 Skadar Lake ...................................................................................................................7 Chironomidae ..............................................................................................................10 Species concept and integrative taxonomy .................................................................12 DNA barcoding ...........................................................................................................14 Chapter I. First insight into the diversity and ecology of non-biting midges (Diptera: Chironomidae) -

CHIRONOMUS NEWSLETTER on CHIRONOMIDAE RESEARCH Co-Editors: Ruth CONTRERAS-LICHTENBERG Naturhistorisches Museum Wien, Burgring 7, A-1014 WIEN, Austria Peter H
CHIRONOMUS NEWSLETTER ON CHIRONOMIDAE RESEARCH Co-Editors: Ruth CONTRERAS-LICHTENBERG Naturhistorisches Museum Wien, Burgring 7, A-1014 WIEN, Austria Peter H. LANGTON 5 Kylebeg Avenue, Mountsandel, Coleraine, Co. Londonderry, Northern Ireland, BT52 1JN - Northern Ireland Bibliography: Odwin HOFFRICHTER Institut f. Biologie I, Albert-Ludwigs-Universität Freiburg, Hauptstrasse 1 D-79104 , Germany Treasurer: Trond ANDERSEN: Museum of Zoology, University of Bergen, Museplass 3, N-5007 Bergen - Norway ISSN 0172-1941 No. 13 September 2000 CONTENTS Chironomid Work in Munich to Continue ............................................................................................................... 1 New curator at the Zoologische Staatssammlung Munich ...................................................................................... 2 Contributions in SPIXIANA in Memory of Dr. Reiss.............................................................................................. 4 To Iya Kiknadze at 70................................................................................................................................................ 5 Current Research ....................................................................................................................................................... 7 Short – Communications ......................................................................................................................................... 19 Notice Board ................................................................................................................................... -
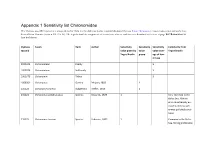
Appendix 1 Sensitivity List Chironomidae the Dyntaxa Taxonid Represents a Unique Identifier from the Swedish Taxonomic Standard Database Dyntaxa (
Appendix 1 Sensitivity list Chironomidae The Dyntaxa taxonID represents a unique identifier from the Swedish taxonomic standard database Dyntaxa (http://dyntaxa.se). Taxon names rank and author are derived from Dyntaxa (version 2013-06-26). The logic behind the assignment of a sensitivity class to each taxon is described in the text at page Fel! Bokmärket är inte definierat.. Dyntaxa Taxon Rank Author Sensitivity Sensitivity Sensitivity Comments from taxonid value given by value value aver- Yngve Brodin Yngve Brodin group age of low- er taxa 2001302 Chironomidae Family 5 1009974 Chironominae Subfamily 5 1009975 Chironomini Tribus 5 1009301 Chironomus Genera Meigen, 1803 1 235223 Camptochironomus Subgenera Kieffer, 1918 1 235224 Chironomus pallidivittatus Species Edwards, 1929 1 Very common in the Baltic Sea. Able to endure extremely eu- trophic and also oth- erwise polluted condi- tions. 235225 Chironomus tentans Species Fabricius, 1805 1 Common in the Baltic Sea. Strong preference WATERS: A PROBABILITY BASED INDEX FOR BENTHIC ASSESSMENT IN THE BALTIC SEA Dyntaxa Taxon Rank Author Sensitivity Sensitivity Sensitivity Comments from taxonid value given by value value aver- Yngve Brodin Yngve Brodin group age of low- er taxa for eutrophic and even extremely eutrophic and polluted condi- tions. 235228 Chironomus Subgenera Meigen, 1803 1 235234 Chironomus annularius Species Meigen, 1818 1 Rather common in the Baltic Sea, easily con- fused with several other Chironomus species bout as larvae and adults. 235235 Chironomus anthracinus Species Zetterstedt, 1860 1 Common north of Åland, otherwise less common in the Baltic Sea. Mainly found below the littoral. Prefers less strongly 2 WATERS: A PROBABILITY BASED INDEX FOR BENTHIC ASSESSMENT IN THE BALTIC SEA Dyntaxa Taxon Rank Author Sensitivity Sensitivity Sensitivity Comments from taxonid value given by value value aver- Yngve Brodin Yngve Brodin group age of low- er taxa eutrophic conditions than C. -

Stable Isotopes Reveal Alternative Energy Pathways in Aquatic
1 2 The incredible lightness of being methane-fuelled: stable isotopes 3 reveal alternative energy pathways in aquatic ecosystems and beyond 4 5 Jonathan Grey 6 Lancaster Environment Centre, Lancaster University, Lancaster, UK. 7 8 Correspondence: 9 Prof. Jonathan Grey 10 Lancaster Environment Centre 11 Bailrigg Campus 12 Lancaster University 13 Lancaster, LA1 4YQ, UK 14 15 [email protected] 16 17 18 Abstract 19 We have known about the processes of methanogenesis and methanotrophy for over 100 20 years, since the days of Winogradsky, yet their contributions to the carbon cycle were 21 deemed to be of negligible importance for the majority of that period. It is only in the last two 22 decades that methane has been appreciated for its role in the global carbon cycle, and stable 23 isotopes have come to the forefront as tools for identifying and tracking the fate of methane- 24 derived carbon within food webs, especially within aquatic ecosystems. While it is not 25 surprising that chemosynthetic processes dominate and contribute almost 100% to the 26 biomass of organisms residing within extreme habitats like deep ocean hydrothermal vents 27 and seeps, way below the reach of photosynthetically active radiation, it is perhaps 28 counterintuitive to find reliance upon methane-derived carbon in shallow, well-lit, well- 29 oxygenated streams. Yet, apparently, methane-derived carbon contributes to varying degrees 30 across the spectrum from point sources to extremely diffuse sources. Certainly a good 31 proportion of the evidence for methane-derived carbon contributing to freshwater food webs 32 comes from somewhere in the middle of that spectrum; from studies of seasonally stratifying 33 lakes (mono- or dimictic) wherein, there is a defined gradient or boundary at which anoxic 34 meet oxic conditions and consequently allows for close coupling of methanogenesis and 35 methanotrophy. -

National Park Service
Communities in Freshwater Coastal Rock Pools of Lake Superior, with a Focus on Chironomidae (Diptera) A Dissertation SUBMITTED TO THE FACULTY OF UNIVERSITY OF MINNESOTA BY Alexander Taurus Egan IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY Advisor: Leonard C. Ferrington, Jr. May 2014 © Alexander Taurus Egan 2014 Acknowledgements Projects of this size are rarely accomplished without the assistance and support of many people. Primarily, my advisor, Len Ferrington, has been a great source of guidance and enthusiasm. My committee, Jacques Finlay, Ralph Holzenthal, and Roger Moon, have raised the bar considerably by pushing, pulling and steering me toward being a better scientist. Friends and colleagues in the Chironomidae Research Group have made my graduate experience a time I will remember fondly, with Alyssa Anderson, Will Bouchard and Jessica Miller sharing in the successes, misfortunes, and minor but important goals that come with the territory. In particular, Petra Kranzfelder often filled the roles of peer advisor and sounding board for ideas both brilliant and ridiculous. The National Park service has been very generous in many ways, and specific thanks go to Brenda Moraska Lafrançois and Jay Glase, who provided early development and direction for this project. My colleagues Mark Edlund from the Science Museum of Minnesota and Toben Lafrançois from the Science Museum and Northland College have consistently offered excellent ecological advice on what the data mean, often acting as de facto advisors. Without support from Isle Royale National Park this project would not have been possible. In particular, the technical advice, equipment loans, and logistical assistance from Paul Brown, Rick Damstra, Joan Elias, and Mark Romanski were invaluable. -

De Eurasian Lakes with Respect to Temperature and Continentality: Development and Application of New Chironomid-Based Climate-Inference Models in Northern Russia
Quaternary Science Reviews 30 (2011) 1122e1141 Contents lists available at ScienceDirect Quaternary Science Reviews journal homepage: www.elsevier.com/locate/quascirev The distribution and abundance of chironomids in high-latitude Eurasian lakes with respect to temperature and continentality: development and application of new chironomid-based climate-inference models in northern Russia A.E. Self a,b,*, S.J. Brooks a, H.J.B. Birks b,c,d, L. Nazarova e, D. Porinchu f, A. Odland g, H. Yang b, V.J. Jones b a Department of Entomology, Natural History Museum, Cromwell Road, London SW7 5BD, UK b Environmental Change Research Centre, Department of Geography, University College London, Gower Street, London WC1E 6BT, UK c Department of Biology, Bjerknes Centre for Climate Research, University of Bergen, P.O. Box 7803, N-5020 Bergen, Norway d School of Geography and the Environment, University of Oxford, South Parks Road, Oxford OX1 3QY, UK e Alfred Wegener Institute for Polar and Marine Research, Telegrafenberg A 43, 14473 Potsdam, Germany f Department of Geography, The Ohio State University, 1036 Derby Hall, 154 N. Oval Mall, Columbus, OH 43210, USA g Institute of Environmental Studies, Telemark University College, N-3800 Bø, Norway article info abstract Article history: The large landmass of northern Russia has the potential to influence global climate through amplification Received 10 June 2010 of climate change. Reconstructing climate in this region over millennial timescales is crucial for Received in revised form understanding the processes that affect the global climate system. Chironomids, preserved in lake 18 January 2011 sediments, have the potential to produce high resolution, low error, quantitative summer air temperature Accepted 19 January 2011 reconstructions. -

Diptera) from Lake Sediments in Central America: a Preliminary Inventory
Zootaxa 4497 (4): 559–572 ISSN 1175-5326 (print edition) http://www.mapress.com/j/zt/ Article ZOOTAXA Copyright © 2018 Magnolia Press ISSN 1175-5334 (online edition) https://doi.org/10.11646/zootaxa.4497.4.6 http://zoobank.org/urn:lsid:zoobank.org:pub:00292B85-305D-44FF-B9D6-DE2D7A72D220 Sub-fossil Chironomidae (Diptera) from lake sediments in Central America: a preliminary inventory LADISLAV HAMERLIK1,2,5, FABIO LAURINDO DA SILVA3,4 & MARTA WOJEWÓDKA1 1Institute of Geological Sciences, Polish Academy of Sciences, Warsaw, Poland 2Department of Biology and Ecology, Matej Bel University, Banská Bystrica, Slovakia 3Laboratory of Systematic and Biogeography of Insecta, Department of Zoology, Institute of Biosciences, University of São Paulo, São Paulo, Brazil. 4Department of Natural History, NTNU University Museum, Norwegian University of Science and Technology, Trondheim, Norway. 5Corresponding author. E-mail: [email protected] Abstract The chironomid diversity of Central America is virtually underestimated and there is almost no knowledge on the chirono- mid remains accumulated in surface sediments of lakes. Thus, in the present study we provide information on the larval sub-fossil chironomid fauna from surface sediments in Central American lakes for the first time. Samples from 27 lakes analysed from Guatemala, El Salvador and Honduras yielded a total of 1,109 remains of four subfamilies. Fifty genera have been identified, containing at least 85 morphospecies. With 45 taxa, Chironominae were the most specious and also most abundant subfamily. Tanypodinae with 14 taxa dominated in about one third of the sites. Orthocladiinae were pre- sented by 24 taxa, but were recorded in 9 sites, being dominant in only one site. -

(Insecta: Diptera) in Lowland Running Waters of North-East Germany (Brandenburg) Based on 10- Year EU-Water Framework Directive Monitoring Programme
37 Lauterbornia 77: 37-62, D-86424 Dinkelscherben, 2014-07-07 Faunistic overview of Chironomidae (Insecta: Diptera) in lowland running waters of north-east Germany (Brandenburg) based on 10- year EU-Water Framework Directive monitoring programme Claus Orendt, Xavier-François Garcia, Berthold F. Janecek, Susanne Michiels, Claus-Joachim Otto and Reinhard Müller With 8 figures and 5 tables Keywords : Chironomidae, Diptera, Insecta, Brandenburg, Germany, Central European Lowlands, running water, check list, faunistics, larva, pupa, pupal exuviae, imago Schlagwörter : Chironomidae, Diptera, Insecta, Brandenburg, Deutschland, Zentrales Flachland, Fließgewässer, Checkliste, Faunistik, Larve, Puppe, Imago The results of a 10-year monitoring programme are used to provide a list of the Chironomidae taxa of running waters in Brandenburg, north-east Germany, Central European Lowlands ecoregion. The 573 taxa recorded, represent 58 % of the Chironomidae in the "Taxa list of the freshwater organisms of Germany". The 408 re- cords with a valid species status include 56 % of the species, and the 121 genera include 73 % of the genera of the German Chironomidae fauna. The records are based on collections of all developmental stages and were de- rived from about 2350 samples taken during a 10-year monitoring programme up to 2013. The study shows how monitoring programmes with a clear strategy and proper data storage and management can make a sub- stantial contribution to knowledge of the fauna of an ecoregion and provide a solid database for ecological ana- lyses. Further data evaluation indicated an affinity of certain taxa to a particular water type, but further in- depth analysis is required. 1 Introduction Until the end of the last century understanding about the distribution of Chironomidae in the northern lowlands of Germany was limited, because they were seldom included in waterbody studies. -

The Larval Chironomidae (Diptera) Fauna of Gökçeada (Imbroz)
G.U. Journal of Science 19(2): 69-75 (2006) www.gujs.org The Larval Chironomidae (Diptera) Fauna of Gökçeada (Imbroz) Nurcan ÖZKAN♣ Trakya University, Education Faculty, Department of Primary Education, 22030, Edirne, TURKEY Received: 04. 10. 2004 Accepted: 16. 09. 2005 ABSTRACT Benthos specimens were collected by hand mud scoop from 21 localities in Gökçeada during each field work, between 06-28.07.1991 and 06-10.08.1999. Specimens were collected from every kind of habitat as possible (e.g. lakes, ponds, dams, streams and troughs). As a result of this study 53 species of 34 genuses were determined from subfamilies Tanypodinae, Orthocladiinae and Chironominae (Chironomini and Tanytarsini) of Chironomidae family. 35 of the 53 species that were determined are new records for the larval Chironomidae fauna of Gökçeada. 25, the species number described in previous studies in Gökçeada, has increased to 60 with this study. Key Words: Diptera, Chironomidae, Limnofauna, Gökçeada (Imbroz), TURKEY. 1. INTRODUCTION 1.1 Description of Work Field 2. MATERIAL and METHOD Gökçeada is in the North east region of the Aegean Benthos specimens were collected with hand mud scoop sea which occurred by the breaking and collapsement of from 21 localities in Gökçeada during each field work, the ground at the end of the 3rd and at the beginning of between 06-28.07.1991 and 06-10.08.1999. They were the 6th geological era, approximately 2-2,5 million years fixed with 70% alcohol and brought to the laboratory ago, and it is the continuity of Anatolia and Thrace [1, 2, (Figure 1).