Mammal Extinctions and the Increasing Isolation of Humans on the Tree of Life Sandrine Pavoine, Michael Bonsall, T
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Population Density of Tarsius Dianae in Central Sulawesi
10 Asian Primates Journal 1(1), 2008 RELATIVE POPULATION DENSITY OF Tarsius dianae IN MAN-INFLUENCED HABITATS OF LORE LINDU NATIONAL PARK, CENTRAL SULAWESI, INDONESIA Indra Yustian1, Stefan Merker2, Jatna Supriatna3, and Noviar Andayani4 1 Dept. of Biology, Faculty of Mathematics and Natural Sciences, University of Sriwijaya, Indonesia. 2 Institute of Anthropology, University of Mainz, Germany. 3 Conservation International Indonesia and Department of Biology, University of Indonesia. 4 Wildlife Conservation Society-Indonesia Program and Department of Biology, University of Indonesia, Indonesia. ABSTRACT The aim of this study was to know the impact of human activities on population density of Tarsius dianae by estimating the relative population density in four habitat types differently influenced by man. The study was conducted in the vicinity of Kamarora, at the northeastern boundary of Lore Lindu National Park, Central Sulawesi. Four different habitats were chosen: (H1) primary or old secondary forest; (H2) secondary forest ± 30 years after clearance with small-scale selective logging; (H3) forest with interspersed small coffee and cocoa plantations; and (H4) forest with selective logging and plantations. The tarsiers’ sleeping sites were determined using triangulation. Relative population density was estimated by measuring the average distances between three nearest neighbors. The results suggest that different human-influenced habitat have different effects on tarsier’s density. The smallest distances (116.2 ± 18 m) between sleeping sites, which represent the highest estimated population density (57.1 groups in one square km), were found in habitat type H1, the least disturbed habitat. Estimated population density in habitat type H3 or “forest plantations” was 38 groups/km2, followed by habitat type H2 or secondary forest with selective logging 36.4 groups/km2, and the smallest population density was estimated at 32.9 groups/km2 in habitat type H4 or forest with selective logging and plantations. -

Nanda Bess Grow
Curriculum Vitae Nanda Bess Grow Department of Sociology, Social Work, and Anthropology Utah State University [email protected] Logan, UT 84322 RESEARCH INTERESTS Evolutionary ecology ∙ Primate evolution and adaptation ∙ Altitudinal variation in behavior and biology ∙ Sexual selection ∙ Human-primate impacts and conservation EDUCATION 2013 Ph.D. Biological Anthropology Dept. of Anthropology, Texas A&M University Dissertation: “Altitudinal Effects on the Behavior and Morphology of Pygmy Tarsiers (Tarsius pumilus) in Central Sulawesi, Indonesia” Chair: S. Gursky 2011 M.A. Biological Anthropology Dept. of Anthropology, Texas A&M University 2004 B.A. Anthropology (Magna Cum Laude), Minor in Mathematics Barrett Honors College and School of Human Evolution & Social Change, Arizona State University Thesis Chair: L. Nash PROFESSIONAL APPOINTMENTS Aug 2017 - present Lecturer Utah State University, Dept. of Sociology, Social Work, and Anthropology 2014 – July 2017 Instructor Utah State University, Dept. of Sociology, Social Work, and Anthropology 2014 Postdoctoral Researcher Oxford Brookes University, School of Social Sciences and Law PUBLICATIONS Refereed Journal Articles Grow NB. (2019). Cryptic Communication in a Montane Nocturnal Haplorhine, Tarsius pumilus. Folia Primatologica 90(5). (pages forthcoming) Gursky S, Salibay C, Grow NB, Fields L. (2017). Impact of Typhoon Haiyan on the Philippine tarsier population. Folia Primatologica 88(4):323-332. Grow NB, Wirdateti, Nekaris A. (2014). Does toxic defense in slow lorises relate to ectoparasites? The lethal effects of slow loris venom on arthropods. Toxicon 95:1-5. Grow NB, Gursky S, Duma Y. (2013). Altitude and Forest Edges Influence the Density and Distribution of Pygmy Tarsiers (Tarsius pumilus). American Journal of Primatology 75(5): 464-477. -

A Radiographic Study of Human-Primate Commensalism
Developments in Primatology: Progress and Prospects Series Editor Russell H. Tuttle Department of Anthropology The University of Chicago For further volumes, go to http://www.springer.com/series/5852 Sharon Gursky-Doyen ● Jatna Supriatna Editors Indonesian Primates Editors Sharon Gursky-Doyen Jatna Supriatna Department of Anthropology Conservation International Indonesia Texas A&M University University of Indonesia College Station, TX Jakarta USA Indonesia [email protected] [email protected] ISBN 978-1-4419-1559-7 e-ISBN 978-1-4419-1560-3 DOI 10.1007/978-1-4419-1560-3 Springer New York Dordrecht Heidelberg London Library of Congress Control Number: 2009942275 © Springer Science+Business Media, LLC 2010 All rights reserved. This work may not be translated or copied in whole or in part without the written permission of the publisher (Springer Science+Business Media, LLC, 233 Spring Street, New York, NY 10013, USA), except for brief excerpts in connection with reviews or scholarly analysis. Use in connection with any form of information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed is forbidden. The use in this publication of trade names, trademarks, service marks, and similar terms, even if they are not identified as such, is not to be taken as an expression of opinion as to whether or not they are subject to proprietary rights. Printed on acid-free paper Springer is part of Springer Science+Business Media (www.springer.com) S.L. Gursky-Doyen dedicates this volume to her parents, Ronnie Bender and Burt Gursky, who after all these years still do not really know what she does, but they proudly display her books on their coffee table; and to her husband Jimmie who taught her what love is. -

Ecology and Behaviour of Tarsius Syrichta in the Wild
O',F Tarsius syrichta ECOLOGY AND BEHAVIOUR - IN BOHOL, PHILIPPINES: IMPLICATIONS FOR CONSERVATION By Irene Neri-Arboleda D.V.M. A thesis submitted in fulfillment of the requirements for the degree of Master of Applied Science Department of Applied and Molecular Ecology University of Adelaide, South Australia 2001 TABLE OF CONTENTS DAge Title Page I Table of Contents............ 2 List of Tables..... 6 List of Figures.... 8 Acknowledgements... 10 Dedication 11 I)eclaration............ t2 Abstract.. 13 Chapter I GENERAL INTRODUCTION... l5 1.1 Philippine Biodiversity ........... t6 1.2 Thesis Format.... l9 1.3 Project Aims....... 20 Chapter 2 REVIEIV OF TARSIER BIOLOGY...... 2t 2.1 History and Distribution..... 22 2.t.1 History of Discovery... .. 22 2.1.2 Distribution...... 24 2.1.3 Subspecies of T. syrichta...... 24 2.2 Behaviour and Ecology.......... 27 2.2.1 Home Ranges. 27 2.2.2 Social Structure... 30 2.2.3 Reproductive Behaviour... 3l 2.2.4 Diet and Feeding Behaviour 32 2.2.5 Locomotion and Activity Patterns. 34 2.2.6 Population Density. 36 2.2.7 Habitat Preferences... ... 37 2.3 Summary of Review. 40 Chapter 3 FßLD SITE AI\D GEIYERAL METHODS.-..-....... 42 3.1 Field Site........ 43 3. 1.1 Geological History of the Philippines 43 3.1.2 Research Area: Corella, Bohol. 44 3.1.3 Physical Setting. 47 3.t.4 Climate. 47 3.1.5 Flora.. 50 3.1.6 Fauna. 53 3.1.7 Human Population 54 t page 3.1.8 Tourism 55 3.2 Methods.. 55 3.2.1 Mapping. -

(Tarsius Pumilus) in CENTRAL SULAWESI, INDONESIA
ALTITUDINAL EFFECTS ON THE BEHAVIOR AND MORPHOLOGY OF PYGMY TARSIERS (Tarsius pumilus) IN CENTRAL SULAWESI, INDONESIA A Dissertation by NANDA BESS GROW Submitted to the Office of Graduate Studies of Texas A&M University in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY Chair of Committee, Sharon Gursky-Doyen Committee Members, Michael Alvard Jeffrey Winking Jane Packard Head of Department, Cynthia Werner August 2013 Major Subject: Anthropology Copyright 2013 Nanda Bess Grow ABSTRACT Pygmy tarsiers (Tarsius pumilus) of Central Sulawesi, Indonesia are the only species of tarsier known to live exclusively at high altitudes. This study was the first to locate and observe multiple groups of this elusive primate. This research tested the hypothesis that variation in pygmy tarsier behavior and morphology correlates with measurable ecological differences that occur along an altitudinal gradient. As a response to decreased resources at higher altitudes and the associated effects on foraging competition and energy intake, pygmy tarsiers were predicted to exhibit lower population density, smaller group sizes, larger home ranges, and reduced sexually selected traits compared to lowland tarsiers. Six groups containing a total of 22 individuals were observed. Pygmy tarsiers were only found between 2000 and 2300 m, indicating allopatric separation from lowland tarsiers. As expected, the observed pygmy tarsiers lived at a lower density than lowland tarsier species, in association with decreased resources at higher altitudes. The estimated population density of pygmy tarsiers was 92 individuals per 100 ha, with 25 groups per 100 ha. However, contrary to expectation, home range sizes were not significantly larger than lowland tarsier home ranges, and average NPL was smaller than those of lowland tarsiers. -

The Distribution and Population Density of Bornean Tarsier, “Tarsius
Tropical Life Sciences Research, 29(1), 139–154, 2018 The Distribution and Population Density of Bornean Tarsier, “Tarsius Bancanus Borneanus (Elliot)” in Secondary and Rehabilitated Forests of Universiti Putra Malaysia, Bintulu Sarawak Campus, Sarawak, Malaysia 1Hani Nabilia Muhd Sahimi, 2John Keen Chubo, 3Marina Mohd. Top @ Mohd. Tah*, 1Noor Bahiah Saripuddin and 1Siti Sarah Ab Rahim ¹Department of Forestry Science, Universiti Putra Malaysia Bintulu Sarawak Campus, 97008 Bintulu, Sarawak, Malaysia ²Department of Crop Science, Universiti Putra Malaysia Bintulu Sarawak Campus, 97008 Bintulu, Sarawak, Malaysia ³Department of Biology, Faculty of Science, Universiti Putra Malaysia, 43400 Serdang, Selangor, Malaysia Published date: 2 March 2018 To cite this article: Hani Nabilia Muhd Sahimi, John Keen Chubo, Marina Mohd. Top @ Mohd. Tah, Noor Bahiah Saripuddin and Siti Sarah Ab Rahim. (2018). The distribution and population density of Bornean Tarsier, “Tarsius Bancanus Borneanus (Elliot)” in secondary and rehabilitated forests of Universiti Putra Malaysia, Bintulu Sarawak Campus, Sarawak, Malaysia. Tropical Life Sciences Research 28(1): 139–154. https://doi.org/10.21315/tlsr2018.29.1.10 To link to this article: https://doi.org/10.21315/tlsr2018.29.1.10 Abstrak: Tarsius bancanus borneanus (kera hantu) pertama kali dicam oleh Elliot pada tahun 1990 merupakan spesies endemik yang boleh ditemui di kepulauan Borneo yang terdiri dari Sabah dan Sarawak (Malaysia), Brunei Darussalam dan Kalimantan, Indonesia. Sub-spesies ini telah disenaraikan sebagai haiwan yang terlindung sepenuhnya di bawah Ordinan Perlindungan Hidupan Liar Sarawak (1998) dan disenaraikan sebagai spesies yang terdedah kepada kepupusan oleh IUCN. Kajian ini telah dijalankan di Universiti Putra Malaysia Kampus Bintulu Sarawak (UPMKB), dari Oktober 2014 hingga Mac 2015. -

Ecology and Conservation Status of Tarsius Bancanus Saltator on Belitung Island, Indonesia
Indra Yustian (Autor) Ecology and Conservation Status of Tarsius bancanus saltator on Belitung Island, Indonesia https://cuvillier.de/de/shop/publications/1802 Copyright: Cuvillier Verlag, Inhaberin Annette Jentzsch-Cuvillier, Nonnenstieg 8, 37075 Göttingen, Germany Telefon: +49 (0)551 54724-0, E-Mail: [email protected], Website: https://cuvillier.de Ecology & Conservation Status of Belitung Tarsier Chapter 1: Introduction 1 Chapter 1: Introduction 1.1 Background Indonesia is one of the most biodiversity-rich and ecologically complex nations in the world. Although covering only 1.3% of the globe, the Indonesian archipelago accounts for nearly 10% of the world’s remaining tropical forest (BAPPENAS 1993), ranked second after Brazil for its forest area and the amount of biodiversity. Despite increasing concern over the loss of tropical forest, significant local and international efforts to find solutions to the problem, and despite the country’s extensive system of protected areas and production forests (forests available for logging), and the abundance of detailed land-use plans, the rate of deforestation in Indonesia continues to increase (Jepson et al. 2001 & Whitten et al. 2001 cited in Kinnaird et al. 2003). Kinnaird et al. (2003) also mentioned that Indonesia provides one mostly relevant example of the devastating effects of enormous deforestation. According to World Bank (2001, cited in USAID/Indonesia 2004), 20 million ha of Indonesia’s forests have been lost at an average annual deforestation rate of 1.5 million ha between 1985 and 1997. Since 1997, the rate of forest lost is 2.4 million ha per year or more. Of about five million ha of forests were degraded by fires in 1997-1998 alone. -

Nanda Bess Grow
Curriculum Vitae Nanda Bess Grow Department of Sociology, Social Work, and Anthropology Utah State University [email protected] Logan, UT 84322 RESEARCH INTERESTS Behavioral ecology ∙ Primate evolution and adaptation ∙ Altitudinal variation in behavior and biology ∙ Island adaptations ∙ Sexual selection EDUCATION 2013 Ph.D. Biological Anthropology Dept. of Anthropology, Texas A&M University Dissertation: “Altitudinal Effects on the Behavior and Morphology of Pygmy Tarsiers (Tarsius pumilus) in Central Sulawesi, Indonesia” Chair: S. Gursky 2011 M.A. Biological Anthropology Dept. of Anthropology, Texas A&M University 2004 B.A. Anthropology (Magna Cum Laude), Minor in Mathematics Barrett Honors College and School of Human Evolution & Social Change, Arizona State University Thesis Chair: L. Nash PROFESSIONAL APPOINTMENTS Aug 2017 - present Lecturer Utah State University, Dept. of Sociology, Social Work, and Anthropology 2014 – July 2017 Instructor Utah State University, Dept. of Sociology, Social Work, and Anthropology 2014 Postdoctoral Researcher Oxford Brookes University, School of Social Sciences and Law PUBLICATIONS Refereed Journal Articles Gursky S, Salibay C, Grow NB, Fields L. (2017). Impact of Typhoon Haiyan on the Philippine tarsier population. Folia Primatologica 88(4):323-332 Grow NB, Wirdateti, Nekaris A. (2014). Does toxic defense in slow lorises relate to ectoparasites? The lethal effects of slow loris venom on arthropods. Toxicon 95:1-5. Grow NB, Gursky S, Duma Y. (2013). Altitude and Forest Edges Influence the Density and Distribution of Pygmy Tarsiers (Tarsius pumilus). American Journal of Primatology 75(5): 464-477. Grow NB, Gursky-Doyen S. (2010). Preliminary Data on the Ecology, Behavior, and Morphology of Tarsius pumilus. International Journal of Primatology 31(6): 1174-1191. -

Primate Families-Short
The Primates compiled by Dana Visalli A male Mandrill Primates arose from ancestors that lived in the trees of tropical forests; many primate characteristics represent adaptations to life in this challenging three-dimensional environment. Most primate species remain at least partly arboreal. There are a surprisingly large number of primates, or ‘monkeys and their kin.’ The total number is variable, but settles in the range of approximately 325 species. The word ‘primate’ comes from a Latin root meaning ‘first rank’ or ‘numeral uno,’ a reflection of ever-present an- thropocentrism (human self-centeredness) of our species, which tends to judge nearby Homo sapiens (which means ‘wise man’) as the pinnacle of the infinitely long evolutionary journey, while those Homo sapiens at a distance are often considered to be ‘the other’ and ‘the enemy’ and therefore not wise at all. Primates range in size from a tiny lemur in Madagascar called Madame Berthe’s Mouse Lemur, which weighs one ounce, to the lowland gorillas in Africa, which weigh in at well over 400 pounds. Genetic studies indicate that the primate line originated about 85 million years ago, in the mid-Cretaceous Peri- od. New primate species are still being discovered. More than 25 species were taxonomically described in the decade of the 2000s and eleven have been described since 2010. Primates are characterized by large brains relative to other mammals, as well as an increased reliance on stereoscopic vision at the expense of smell, the dominant sensory system in most mammals. These features are more developed in monkeys and apes and noticeably less so in lorises and lemurs. -
Species on IUCN
KIDS CORNER SPECIES & IUCN This document aims to provide you with an overview of the International Union for the Conservation of Nature (IUCN). This presentation has the following structure: Slide 1 - What is the IUCN? Slide 2 - Extinct (EX) Slide 3 - Case Study – Tasmanian Tiger Slide 4 - Extinct in the Wild (EW) Slide 5 - Critically Endangered (CE) Slide 6 - Endangered (EN) Slide 7 - Vulnerable (VU) Slide 8 - Near Threatened (NT) Slide 9 - Least Concern (LC) Slide 10 - Data Deficient (DD) Slide 11 - Australian Curriculum Mapping KIDS CORNER SPECIES & IUCN What is the IUCN? The IUCN Red List of Threatened Species is the world's most comprehensive inventory of the global conservation status of plant and animal species. Species are constantly being moved into different criteria depending on the results from regular scientific studies. Species are classified by the IUCN Red List into nine groups, set through criteria such as rate of decline, population siZe, area of geographic distribution, and degree of population and distribution fragmentation. Here is a list of the categories and some interesting and unusual species that feature in each group: • Extinct (EX) • Extinct in the Wild (EW) • Critically Endangered (CR) • Endangered (EN) • Vulnerable (VU) • Near Threatened (NT) • Least Concern (LC) • Data Deficient (DD) KIDS CORNER SPECIES & IUCN Extinct (EX) – No known individuals remaining The Tasmanian tiger, also known as the thylacine, resembled a large, long dog with stripes, a heavy, stiff tail, and a big head. Its scientific name, Thylacinus cynocephalus, means ‘pouched dog with a wolf's head’. Fully grown, it measured about 180 cm from nose to tail tip, stood approximately 58 cm high at the shoulder, and weighed up to 30 kg. -
The World's 25 Most Endangered Primates 2012–2014
Front cover photo: Male Tonkin snub-nosed monkey Rhinopithecus avunculus © Le Van Dung Primates in Peril: The World’s 25 Most Endangered Primates 2012–2014 Edited by Christoph Schwitzer, Russell A. Mittermeier, Anthony B. Rylands, Lucy A. Taylor, Federica Chiozza, Elizabeth A. Williamson, Janette Wallis and Fay E. Clark Illustrations by Stephen D. Nash IUCN SSC Primate Specialist Group (PSG) International Primatological Society (IPS) Conservation International (CI) Bristol Zoological Society (BZS) This publication was supported by the Margot Marsh Biodiversity Foundation and the Humane Society International. Published by: IUCN SSC Primate Specialist Group (PSG), International Primatological Society (IPS), Conservation International (CI), Bristol Zoological Society (BZS) Copyright: ©2014 Conservation International All rights reserved. No part of this report may be reproduced in any form or by any means without permission in writing from the publisher. Inquiries to the publisher should be directed to the following address: Russell A. Mittermeier, Chair, IUCN SSC Primate Specialist Group, Conservation International, 2011 Crystal Drive, Suite 500, Arlington, VA 22202, USA. Citation (report): Schwitzer, C., Mittermeier, R. A., Rylands, A. B., Taylor, L. A., Chiozza, F., Williamson, E. A., Wallis, J. and Clark, F. E. (eds.). 2014. Primates in Peril: The World’s 25 Most Endangered Primates 2012–2014. IUCN SSC Primate Specialist Group (PSG), International Primatological Society (IPS), Conservation International (CI), and Bristol Zoological Society, Arlington, VA. iv+87pp. Citation (species): Mbora, D. N. M. and Butynski, T. M. 2014. Tana River red colobus Piliocolobus rufomitratus (Peters, 1879). In: C. Schwitzer, R. A. Mittermeier, A. B. Rylands, L. A. Taylor, F. Chiozza, E. A. Williamson, J. -
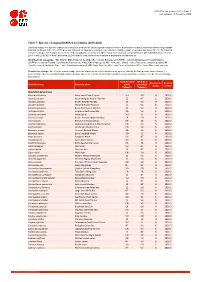
Table 7: Species Changing IUCN Red List Status (2019-2020)
IUCN Red List version 2020-3: Table 7 Last Updated: 10 December 2020 Table 7: Species changing IUCN Red List Status (2019-2020) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2019 (IUCN Red List version 2019-3) and 2020 (IUCN Red List version 2020-3) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered [CR(PE) - Critically Endangered (Possibly Extinct), CR(PEW) - Critically Endangered (Possibly Extinct in the Wild)], EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.); E - Previous listing was an Error. IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2019) List (2020) change version Category