Topic 1. Elements, Compounds and Mixtures
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hendrickson SP.Pdf
CHEMISTRY IN THE WORLD THIRD EDITION BY Dr. Kirstin Hendrickson ARIZONA STATE UNIVERSITY Bassim Hamadeh, CEO and Publisher Kassie Graves, Director of Acquisitions Jamie Giganti, Senior Managing Editor Jess Estrella, Senior Graphic Designer Bob Farrell, Senior Field Acquisitions Editor Natalie Lakosil, Licensing Manager Kaela Martin, Allie Kiekhofer, and Rachel Singer, Associate Editors Kat Ragudos, Interior Designer Copyright © 2017 by Cognella, Inc. All rights reserved. No part of this publication may be reprinted, reproduced, transmitted, or utilized in any form or by any electronic, mechanical, or other means, now known or hereafter invented, including photocopying, microfilming, and recording, or in any information retrieval system without the written permission of Cognella, Inc. Trademark Notice: Product or corporate names may be trademarks or registered trademarks, and are used only for identification and explanation without intent to infringe. Cover image copyright © Scott Lefler. copyright © Scott Lefler. copyright © Scott Lefler. copyright © 2011 Depositphotos/Nadezda Razvodovska. Printed in the United States of America ISBN: 978-1-63487-540-0 (pbk) / 978-1-63487-541-7 (br) For my students, from whom I have learned more than I could ever teach. DEDICATION CONTENTS Acknowledgments xi To the Student and the Instructor: xiii How to Use This Book UNIT 1: ALL AROUND US AND INSIDE OF US 1 Chapter 1: Chemistry Is Everywhere 3 1.1 Classifications of Matter 5 1.2 The Periodic Table 12 1.3 Thinking About Chemicals, Chemical 14 Reactions, -

2. Molecular Stucture/Basic Spectroscopy the Electromagnetic Spectrum
2. Molecular stucture/Basic spectroscopy The electromagnetic spectrum Spectral region fooatocadr atomic and molecular spectroscopy E. Hecht (2nd Ed.) Optics, Addison-Wesley Publishing Company,1987 Per-Erik Bengtsson Spectral regions Mo lecu lar spec troscopy o ften dea ls w ith ra dia tion in the ultraviolet (UV), visible, and infrared (IR) spectltral reg ions. • The visible region is from 400 nm – 700 nm • The ultraviolet region is below 400 nm • The infrared region is above 700 nm. 400 nm 500 nm 600 nm 700 nm Spectroscopy: That part of science which uses emission and/or absorption of radiation to deduce atomic/molecular properties Per-Erik Bengtsson Some basics about spectroscopy E = Energy difference = c /c h = Planck's constant, 6.63 10-34 Js ergy nn = Frequency E hn = h/hc /l E = h = hc / c = Velocity of light, 3.0 108 m/s = Wavelength 0 Often the wave number, , is used to express energy. The unit is cm-1. = E / hc = 1/ Example The energy difference between two states in the OH-molecule is 35714 cm-1. Which wavelength is needed to excite the molecule? Answer = 1/ =35714 cm -1 = 1/ = 280 nm. Other ways of expressing this energy: E = hc/ = 656.5 10-19 J E / h = c/ = 9.7 1014 Hz Per-Erik Bengtsson Species in combustion Combustion involves a large number of species Atoms oxygen (O), hydrogen (H), etc. formed by dissociation at high temperatures Diatomic molecules nitrogen (N2), oxygen (O2) carbon monoxide (CO), hydrogen (H2) nitr icoxide (NO), hy droxy l (OH), CH, e tc. -

Toxic Metals in the Environment: the Role of Surfaces
Toxic Metals in the Environment: The Role of Surfaces Donald L. Sparks1 etals are prevalent in the environment. They are derived from both such as density, weight, atomic number, and degree of toxicity natural and anthropogenic sources. Certain metals are essential for (Roberts et al. 2005). Certain met- Mplant growth and for animal and human health. However, if present als and metalloids are essential for in excessive concentrations they become toxic. Metals undergo an array of plant growth and for animal and human health. With respect to biogeochemical processes at reactive natural surfaces, including surfaces of plants, these are referred to as clay minerals, metal oxides and oxyhydroxides, humic substances, plant roots, micronutrients and include B, Cu, and microbes. These processes control the solubility, mobility, bioavailability, Fe, Zn, Mn, and Mo. In addition, and toxicity of metals in the environment. The use of advanced analytical As, Co, Cr, Ni, Se, Sn, and V are essential in animal nutrition. techniques has furthered our understanding of the reactivity and mobility Micronutrients are also referred to of metals in the near-surface environment. as trace elements since they are required in only small quantities, Keywords: critical zone, metals, sorption, surface complexation, biogeochemical processes unlike major nutrients such as N, P, and K. In excess, trace elements INTRODUCTION can be toxic to plants, microbes, animals, and humans. Metals comprise about 75% of the known elements and can Problems also arise when there is a deficiency in essential form alloys with each other and with nonmetals (Morris elements. 1992). Metals have useful properties such as strength, mal- Important trace elements in the environment are As, Ag, B, leability, and conductivity of heat and electricity. -

Ijmp.Jor.Br V
INDEPENDENT JOURNAL OF MANAGEMENT & PRODUCTION (IJM&P) http://www.ijmp.jor.br v. 10, n. 8, Special Edition Seng 2019 ISSN: 2236-269X DOI: 10.14807/ijmp.v10i8.1046 A NEW HYPOTHESIS ABOUT THE NUCLEAR HYDROGEN STRUCTURE Relly Victoria Virgil Petrescu IFToMM, Romania E-mail: [email protected] Raffaella Aversa University of Naples, Italy E-mail: [email protected] Antonio Apicella University of Naples, Italy E-mail: [email protected] Taher M. Abu-Lebdeh North Carolina A and T State Univesity, United States E-mail: [email protected] Florian Ion Tiberiu Petrescu IFToMM, Romania E-mail: [email protected] Submission: 5/3/2019 Accept: 5/20/2019 ABSTRACT In other papers already presented on the structure and dimensions of elemental hydrogen, the elementary particle dynamics was taken into account in order to be able to determine the size of the hydrogen. This new work, one comes back with a new dynamic hypothesis designed to fundamentally change again the dynamic particle size due to the impulse influence of the particle. Until now it has been assumed that the impulse of an elementary particle is equal to the mass of the particle multiplied by its velocity, but in reality, the impulse definition is different, which is derived from the translational kinetic energy in a rapport of its velocity. This produces an additional condensation of matter in its elemental form. Keywords: Particle structure; Impulse; Condensed matter. [http://creativecommons.org/licenses/by/3.0/us/] Licensed under a Creative Commons Attribution 3.0 United States License 1749 INDEPENDENT JOURNAL OF MANAGEMENT & PRODUCTION (IJM&P) http://www.ijmp.jor.br v. -
Chemical Formulas the Elements
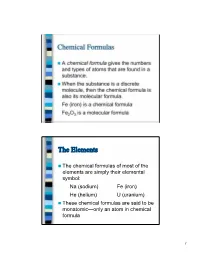
Chemical Formulas A chemical formula gives the numbers and types of atoms that are found in a substance. When the substance is a discrete molecule, then the chemical formula is also its molecular formula. Fe (iron) is a chemical formula Fe2O3 is a molecular formula The Elements The chemical formulas of most of the elements are simply their elemental symbol: Na (sodium) Fe (iron) He (helium) U (uranium) These chemical formulas are said to be monatomic—only an atom in chemical formula 1 The Elements There are seven elements that occur naturally as diatomic molecules—molecules that contain two atoms: H2 (hydrogen) N2 (nitrogen) O2 (oxygen) F2 (fluorine) Cl2 (chlorine) Br2 (bromine) I2 (iodine) The last four elements in this list are in the same family of the Periodic Table Binary Compounds A binary compound is one composed of only two different types of atoms. Rules for binary compound formulas 1. Element to left in Periodic Table comes first except for hydrogen: KCl PCl3 Al2S3 Fe3O4 2 Binary Compounds 2. Hydrogen comes last unless other element is from group 16 or 17: LiH, NH3, B2H4, CH4 3. If both elements are from the same group, the lower element comes first: SiC, BrF3 Other Compounds For compounds with three or more elements that are not ionic, if it contains carbon, this comes first followed by hydrogen. Other elements are then listed in alphabetical order: C2H6O C4H9BrO CH3Cl C8H10N4O2 3 Other Compounds However, the preceding rule is often ignored when writing organic formulas (molecules containing carbon, hydrogen, and maybe other elements) in order to give a better idea of how the atoms are connected: C2H6O is the molecular formula for ethanol, but nobody ever writes it this way—instead the formula is written C2H5OH to indicate one H atom is connected to the O atom. -

Carbon and Silicon
INTERCHAPTER M Carbon and Silicon Carbon nanotubes have potential applications ranging from new composites for use in ultralight automobiles and bullet-proof clothing to ultrathin wires. The discovery of carbon nanotubes helped launch the newly emerging field of nanotechnology. University Science Books, ©2011. All rights reserved. www.uscibooks.com M. CARBON AND siLICON M1 Carbon is widely distributed in nature both as the free M-1. Diamond and Graphite Are Allotropic element and in compounds. The great majority of nat- Forms of Carbon urally occurring carbon is found in coal, petroleum, As discussed in Section 15-10, the two most impor- limestone, CaCO3(s), dolomite, MgCa(CO3)2(s), and a few other deposits. Carbon is also a principal ele- tant allotropic forms of solid carbon are diamond ment in all living matter, and the study of its com- and graphite. Recall that diamond has an extended, pounds forms the vast fields of organic chemistry covalently bonded tetrahedral structure and that and biochemistry. Carbon is decidedly nonmetallic, graphite has a layered structure. Because diamond whereas silicon is a semimetal. is the hardest naturally occurring mineral known, it Silicon constitutes 28% of the mass of the earth’s is extensively used as an abrasive and cutting mate- mantle and is the second most abundant element in rial where a very high resistance to wear is required. the mantle, exceeded only by oxygen. Silicon does not Graphite, however, is the stable form of carbon at occur as the free element in nature; it occurs primar- ordinary temperatures and pressures. It exists as a solid at a higher temperature than any other material ily as silicon dioxide, SiO2(s) and in numerous sili- cates. -

Syllabus of Biochemistry (Hons.)
Syllabus of Biochemistry (Hons.) for SEM-I & SEM-II under CBCS (to be effective from Academic Year: 2017-18) The University of Burdwan Burdwan, West Bengal 1 1. Introduction The syllabus for Biochemistry at undergraduate level using the Choice Based Credit system has been framed in compliance with model syllabus given by UGC. The main objective of framing this new syllabus is to give the students a holistic understanding of the subject giving substantial weight age to both the core content and techniques used in Biochemistry. The ultimate goal of the syllabus is that the students at the end are able to secure a job. Keeping in mind and in tune with the changing nature of the subject, adequate emphasis has been given on new techniques of mapping and understanding of the subject. The syllabus has also been framed in such a way that the basic skills of subject are taught to the students, and everyone might not need to go for higher studies and the scope of securing a job after graduation will increase. It is essential that Biochemistry students select their general electives courses from Chemistry, Physics, Mathematics and/or any branch of Life Sciences disciplines. While the syllabus is in compliance with UGC model curriculum, it is necessary that Biochemistry students should learn “Basic Microbiology” as one of the core courses rather than as elective while. Course on “Concept of Genetics” has been moved to electives. Also, it is recommended that two elective courses namely Nutritional Biochemistry and Advanced Biochemistry may be made compulsory. 2 Type of Courses Number of Courses Course type Description B. -

GANPAT UNIVERSITY FACULTY of SCIENCE TEACHING and EXAMINATION SCHEME Programme Bachelor of Science Branch/Spec
GANPAT UNIVERSITY FACULTY OF SCIENCE TEACHING AND EXAMINATION SCHEME Programme Bachelor of Science Branch/Spec. Microbiology Semester I Effective from Academic Year 2015- Effective for the batch Admitted in June 2015 16 Teaching scheme Examination scheme (Marks) Subject Subject Name Credit Hours (per week) Theory Practical Code Lecture(DT) Practical(Lab.) Lecture(DT) Practical(Lab.) CE SEE Total CE SEE Total L TU Total P TW Total L TU Total P TW Total UMBA101FOM FUNDAMENTALS 04 - 04 - - - 04 - 04 - - - 40 60 100 - - - OF MICROBIOLOGY UCHA101GCH GENERAL 04 - 04 - - - 04 - 04 - - - 40 60 100 - - - CHEMISTRY-I UPHA101GPH GENERAL 04 - 04 - - - 04 - 04 - - - 40 60 100 - - - PHYSICS-I UENA101ENG ENGLISH-I 02 - 02 - - - 02 - 02 - - - 40 60 100 - - - OPEN SUBJECT – 1 02 - 02 - - - 02 - 02 - - - 40 60 100 - - - UPMA101PRA PRACTICAL - - - 02 - 02 - - - 04 - 04 - - - - 50 50 MODULE-I UPCA101PRA PRACTICAL - - - 02 - 02 - - - 04 - 04 - - - - 50 50 MODULE-I UPPA101PRA PRACTICAL - - - 02 - 02 - - - 04 - 04 - - - - 50 50 MODULE-I Total 16 - 16 06 - 06 16 - 16 12 - 12 200 300 500 - 150 150 GANPAT UNIVERSITY FACULTY OF SCIENCE Programme Bachelor of Science Branch/Spec. Microbiology Semester I Version 1.0.1.0 Effective from Academic Year 2015-16 Effective for the batch Admitted in July 2015 Subject code UMBA 101 Subject Name FUNDAMENTALS OF MICROBIOLOGY FOM Teaching scheme Examination scheme (Marks) (Per week) Lecture(DT) Practical(Lab.) Total CE SEE Total L TU P TW Credit 04 -- -- -- 04 Theory 40 60 100 Hours 04 -- -- -- 04 Practical -- -- -- Pre-requisites: Students should have basic knowledge of Microorganisms and microscopy of 10+2 level. Learning Outcome: The course will help the student to understand basic fundamentals of Microbiology and history of Microbiology. -

Chemistry in One Dimension Pierre-Fran¸Coisloos,1, A) Caleb J
Chemistry in One Dimension Pierre-Fran¸coisLoos,1, a) Caleb J. Ball,1 and Peter M. W. Gill1, b) Research School of Chemistry, Australian National University, Canberra ACT 0200, Australia We report benchmark results for one-dimensional (1D) atomic and molecular sys- tems interacting via the Coulomb operator jxj−1. Using various wavefunction-type approaches, such as Hartree-Fock theory, second- and third-order Møller-Plesset per- turbation theory and explicitly correlated calculations, we study the ground state of atoms with up to ten electrons as well as small diatomic and triatomic molecules containing up to two electrons. A detailed analysis of the 1D helium-like ions is given and the expression of the high-density correlation energy is reported. We report the total energies, ionization energies, electron affinities and other interesting properties of the many-electron 1D atoms and, based on these results, we construct the 1D analog of Mendeleev's periodic table. We find that the 1D periodic table contains only two groups: the alkali metals and the noble gases. We also calculate the dissociation + 2+ 3+ curves of various 1D diatomics and study the chemical bond in H2 , HeH , He2 , + 2+ H2, HeH and He2 . We find that, unlike their 3D counterparts, 1D molecules are + primarily bound by one-electron bonds. Finally, we study the chemistry of H3 and we discuss the stability of the 1D polymer resulting from an infinite chain of hydrogen atoms. PACS numbers: 31.15.A-, 31.15.V-, 31.15.xt Keywords: one-dimensional atom; one-dimensional molecule; one-dimensional polymer; helium-like ions arXiv:1406.1343v1 [physics.chem-ph] 5 Jun 2014 a)Electronic mail: Corresponding author: [email protected] b)Electronic mail: [email protected] 1 I. -

Chemical Element
Федеральное государственное бюджетное образовательное учреждение высшего образования «Иркутский государственный медицинский университет» Министерства здравоохранения Российской Федерации Кафедра иностранных языков с курсами латинского языка и русского как иностранного М.О. Наймушина CHEMICAL ELEMENT Учебное пособие Иркутск ИГМУ 2017 УДК 615.011 (075.8)=111 ББК 24.12я73 Н 12 Рекомендовано ЦКМС ФГБОУ ВО ИГМУ Минздрава России в качестве учебного пособия для обучающихся по основным профессиональным образовательным программам высшего образования по специальности «Фармация», при изучении дисциплины «Иностранный (английский) язык» (протокол № 1 от 12.10.2017) М.О. Наймушина - старший преподаватель кафедры иностранных языков с курсами латинского языка и русского как иностранного ФГБОУ ВО ИГМУ Минздрава России Рецензенты: Е.Г. Горячкина – канд. фарм. наук, доцент кафедры фармакогнозии и ботаники ФГБОУ ВО ИГМУ Минздрава России В.В. Литвиненко - к.филол.н., доцент кафедры общеобразовательных дисциплин Байкальского гуманитарного института Наймушина, М.О. Н 12 Chemical Element: учебное пособие / М.О. Наймушина; ФГБОУ ВО ИГМУ Минздрава России, кафедра иностранных языков с курсами латинского языка и русского как иностранного. – Иркутск: ИГМУ, 2017. – 50 с. Учебное пособие включает в себя сокращенные адаптированные тексты на английском языке, сопровождаемые системой лексико-грамматических упражнений и коммуникативных заданий, предназначено для развития навыков различных видов чтения, перевода, аннотирования статей, а также для развития умений монологического -

Laser Cooling and Slowing of a Diatomic Molecule John F
Abstract Laser cooling and slowing of a diatomic molecule John F. Barry 2013 Laser cooling and trapping are central to modern atomic physics. It has been roughly three decades since laser cooling techniques produced ultracold atoms, leading to rapid advances in a vast array of fields and a number of Nobel prizes. Prior to the work presented in this thesis, laser cooling had not yet been extended to molecules because of their complex internal structure. However, this complex- ity makes molecules potentially useful for a wide range of applications. The first direct laser cooling of a molecule and further results we present here provide a new route to ultracold temperatures for molecules. In particular, these methods bridge the gap between ultracold temperatures and the approximately 1 kelvin temperatures attainable with directly cooled molecules (e.g. with cryogenic buffer gas cooling or decelerated supersonic beams). Using the carefully chosen molecule strontium monofluoride (SrF), decays to unwanted vibrational states are suppressed. Driving a transition with rotational quantum number R=1 to an excited state with R0=0 eliminates decays to unwanted ro- tational states. The dark ground-state Zeeman sublevels present in this specific scheme are remixed via a static magnetic field. Using three lasers for this scheme, a given molecule should undergo an average of approximately 100; 000 photon absorption/emission cycles before being lost via un- wanted decays. This number of cycles should be sufficient to load a magneto-optical trap (MOT) of molecules. In this thesis, we demonstrate transverse cooling of an SrF beam, in both Doppler and a Sisyphus-type cooling regimes. -

AP Chemistry!!! Summer Assignment Inside This Packer You Will Find A
Welcome to AP Chemistry!!! Mr. Seck Summer Assignment Inside this packer you will find a summary of chapters 1, 2 and 3 of your textbook. Please read the summary of each topic before answering the questions that follow in each chapter and check your answers in the packer. A good understanding of these topics will help you in further topics. Enjoy working on your summer packet. Have a wonderful summer!!! Thank you 1 Introduction: Matter and Measurement Most of this material was probably learned in first-year chemistry. Quickly review the differences between elements, compounds and mixtures. The separation of mixtures based on their physical properties is important and so are SI units for measurement. Significant figures and dimensional analysis are also introduced here. Pay particular attention to these sections: 1.2 Classification of Matter 1.3 Properties of Matter 1.4 Units of Measurement 1.5 Uncertainty in Measurement 1.6 Dimensional Analysis Classification of Matter Section 1.2 Matter is classified as pure substances or mixtures. Pure substances are either elements or compounds. An element is a substance all of whose atoms contain the same number of protons. A compound is a relatively stable combination of two or more chemically bonded elements in a specific ratio. Mixtures consist of two or more substances. Homogeneous mixtures are uniform throughout. Air, sea water and a nickel coin (a mixture of copper and nickel metals called an alloy) are examples. Heterogeneous mixtures vary in texture and appearance throughout the sample. Rocks, wood, polluted air and muddy water are examples. 2 Section 1.3 Properties of Matter Physical properties can be measured without changing the identity or composition of the substance.