2. Molecular Stucture/Basic Spectroscopy the Electromagnetic Spectrum
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Atomic and Molecular Laser-Induced Breakdown Spectroscopy of Selected Pharmaceuticals
Article Atomic and Molecular Laser-Induced Breakdown Spectroscopy of Selected Pharmaceuticals Pravin Kumar Tiwari 1,2, Nilesh Kumar Rai 3, Rohit Kumar 3, Christian G. Parigger 4 and Awadhesh Kumar Rai 2,* 1 Institute for Plasma Research, Gandhinagar, Gujarat-382428, India 2 Laser Spectroscopy Research Laboratory, Department of Physics, University of Allahabad, Prayagraj-211002, India 3 CMP Degree College, Department of Physics, University of Allahabad, Pragyagraj-211002, India 4 Physics and Astronomy Department, University of Tennessee, University of Tennessee Space Institute, Center for Laser Applications, 411 B.H. Goethert Parkway, Tullahoma, TN 37388-9700, USA * Correspondence: [email protected]; Tel.: +91-532-2460993 Received: 10 June 2019; Accepted: 10 July 2019; Published: 19 July 2019 Abstract: Laser-induced breakdown spectroscopy (LIBS) of pharmaceutical drugs that contain paracetamol was investigated in air and argon atmospheres. The characteristic neutral and ionic spectral lines of various elements and molecular signatures of CN violet and C2 Swan band systems were observed. The relative hardness of all drug samples was measured as well. Principal component analysis, a multivariate method, was applied in the data analysis for demarcation purposes of the drug samples. The CN violet and C2 Swan spectral radiances were investigated for evaluation of a possible correlation of the chemical and molecular structures of the pharmaceuticals. Complementary Raman and Fourier-transform-infrared spectroscopies were used to record the molecular spectra of the drug samples. The application of the above techniques for drug screening are important for the identification and mitigation of drugs that contain additives that may cause adverse side-effects. Keywords: paracetamol; laser-induced breakdown spectroscopy; cyanide; carbon swan bands; principal component analysis; Raman spectroscopy; Fourier-transform-infrared spectroscopy 1. -

Nitrogen-15 Magnetic Resonance Spectroscopy, I
VOL. 51, 1964 CHEMISTRY: LAMBERT, BINSCH, AND ROBERTS 735 11 Felsenfeld, G., G. Sandeen, and P. von Hippel, these PROCEEDINGS, 50, 644 (1963). 12Bollum, F. J., J. Cell. Comp. Physiol., 62 (Suppi. 1), 61 (1963); Von Borstel, R. C., D. M. Prescott, and F. J. Bollum, J. Cell Biol., 19, 72A (1963). 13 Huang, R. C., and J. Bonner, these PROCEEDINGS, 48, 216 (1962); Allfrey, V. G., V. C. Littau, and A. E. Mirsky, these PROCEEDINGS, 49, 414 (1963). 14 Baxill, G. W., and J. St. L. Philpot, Biochim. Biophys. Acta, 76, 223 (1963). NITROGEN-15 MAGNETIC RESONANCE SPECTROSCOPY, I. CHEMICAL SHIFTS* BY JOSEPH B. LAMBERT, GERHARD BINSCH, AND JOHN D. ROBERTS GATES AND CRELLIN LABORATORIES OF CHEMISTRY, t CALIFORNIA INSTITUTE OF TECHNOLOGY Communicated March 23, 1964 Except for the original determination' of nuclear moments, nitrogen magnetic resonance spectroscopy has been limited to the isotope of mass number 14. Al- though N14 is an abundant isotope, it possesses an electric quadrupole moment, which seriously broadens the resonances of nitrogen in all but the most sym- metrical of environments.2 Consequently, nitrogen n.m.r. spectroscopy has seen only limited use in the determination of organic structure. It might be expected that N15, which has a spin of 1/2 and no quadrupole moment, would be very useful, but the low natural abundance (0.36%) and the inherently low signal intensity (1.04 X 10-3 that of H' at constant field) have thus far precluded utilization of N15 in n.m.r. spectroscopy'3 Resonance signals from N'5 have now been obtained from a series of N"5-enriched (30-99%) compounds with a Varian model 4300B spectrometer operated at 6.08 Mc/sec and 14,100 gauss. -

The Power of Crowding for the Origins of Life
Orig Life Evol Biosph (2014) 44:307–311 DOI 10.1007/s11084-014-9382-5 ORIGIN OF LIFE The Power of Crowding for the Origins of Life Helen Greenwood Hansma Received: 2 October 2014 /Accepted: 2 October 2014 / Published online: 14 January 2015 # Springer Science+Business Media Dordrecht 2015 Abstract Molecular crowding increases the likelihood that life as we know it would emerge. In confined spaces, diffusion distances are shorter, and chemical reactions produce fewer and more regular products. Crowding will occur in the spaces between Muscovite mica sheets, which has many advantages as a site for life’s origins. Keywords Muscovite mica . Molecular crowding . Origin of life . Mechanochemistry. Abiogenesis . Chemical confinement effects . Chirality. Protocells Cells are crowded. Protein molecules in cells are typically so close to each other that there is room for only one protein molecule between them (Phillips, Kondev et al. 2008). This is nothing like a dilute ‘prebiotic soup.’ Therefore, by analogy with living cells, the origins of life were probably also crowded. Molecular Confinement Effects Many chemical reactions are limited by the time needed for reactants to diffuse to each other. Shorter distances speed up these reactions. Molecular complementarity is another principle of life in which pairs or groups of molecules form specific interactions (Root-Bernstein 2012). Current examples are: enzymes & substrates & cofactors; nucleic acid base pairs; antigens & antibodies; nucleic acid - protein interactions. Molecular complementarity is likely to have been involved at life’s origins and also benefits from crowding. Mineral surfaces are a likely place for life’s origins and for formation of polymeric molecules (Orgel 1998). -

Ijmp.Jor.Br V
INDEPENDENT JOURNAL OF MANAGEMENT & PRODUCTION (IJM&P) http://www.ijmp.jor.br v. 10, n. 8, Special Edition Seng 2019 ISSN: 2236-269X DOI: 10.14807/ijmp.v10i8.1046 A NEW HYPOTHESIS ABOUT THE NUCLEAR HYDROGEN STRUCTURE Relly Victoria Virgil Petrescu IFToMM, Romania E-mail: [email protected] Raffaella Aversa University of Naples, Italy E-mail: [email protected] Antonio Apicella University of Naples, Italy E-mail: [email protected] Taher M. Abu-Lebdeh North Carolina A and T State Univesity, United States E-mail: [email protected] Florian Ion Tiberiu Petrescu IFToMM, Romania E-mail: [email protected] Submission: 5/3/2019 Accept: 5/20/2019 ABSTRACT In other papers already presented on the structure and dimensions of elemental hydrogen, the elementary particle dynamics was taken into account in order to be able to determine the size of the hydrogen. This new work, one comes back with a new dynamic hypothesis designed to fundamentally change again the dynamic particle size due to the impulse influence of the particle. Until now it has been assumed that the impulse of an elementary particle is equal to the mass of the particle multiplied by its velocity, but in reality, the impulse definition is different, which is derived from the translational kinetic energy in a rapport of its velocity. This produces an additional condensation of matter in its elemental form. Keywords: Particle structure; Impulse; Condensed matter. [http://creativecommons.org/licenses/by/3.0/us/] Licensed under a Creative Commons Attribution 3.0 United States License 1749 INDEPENDENT JOURNAL OF MANAGEMENT & PRODUCTION (IJM&P) http://www.ijmp.jor.br v. -
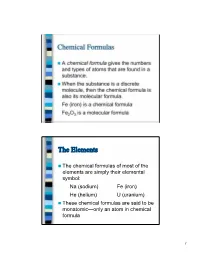
Chemical Formulas the Elements
Chemical Formulas A chemical formula gives the numbers and types of atoms that are found in a substance. When the substance is a discrete molecule, then the chemical formula is also its molecular formula. Fe (iron) is a chemical formula Fe2O3 is a molecular formula The Elements The chemical formulas of most of the elements are simply their elemental symbol: Na (sodium) Fe (iron) He (helium) U (uranium) These chemical formulas are said to be monatomic—only an atom in chemical formula 1 The Elements There are seven elements that occur naturally as diatomic molecules—molecules that contain two atoms: H2 (hydrogen) N2 (nitrogen) O2 (oxygen) F2 (fluorine) Cl2 (chlorine) Br2 (bromine) I2 (iodine) The last four elements in this list are in the same family of the Periodic Table Binary Compounds A binary compound is one composed of only two different types of atoms. Rules for binary compound formulas 1. Element to left in Periodic Table comes first except for hydrogen: KCl PCl3 Al2S3 Fe3O4 2 Binary Compounds 2. Hydrogen comes last unless other element is from group 16 or 17: LiH, NH3, B2H4, CH4 3. If both elements are from the same group, the lower element comes first: SiC, BrF3 Other Compounds For compounds with three or more elements that are not ionic, if it contains carbon, this comes first followed by hydrogen. Other elements are then listed in alphabetical order: C2H6O C4H9BrO CH3Cl C8H10N4O2 3 Other Compounds However, the preceding rule is often ignored when writing organic formulas (molecules containing carbon, hydrogen, and maybe other elements) in order to give a better idea of how the atoms are connected: C2H6O is the molecular formula for ethanol, but nobody ever writes it this way—instead the formula is written C2H5OH to indicate one H atom is connected to the O atom. -

Syllabus of Biochemistry (Hons.)
Syllabus of Biochemistry (Hons.) for SEM-I & SEM-II under CBCS (to be effective from Academic Year: 2017-18) The University of Burdwan Burdwan, West Bengal 1 1. Introduction The syllabus for Biochemistry at undergraduate level using the Choice Based Credit system has been framed in compliance with model syllabus given by UGC. The main objective of framing this new syllabus is to give the students a holistic understanding of the subject giving substantial weight age to both the core content and techniques used in Biochemistry. The ultimate goal of the syllabus is that the students at the end are able to secure a job. Keeping in mind and in tune with the changing nature of the subject, adequate emphasis has been given on new techniques of mapping and understanding of the subject. The syllabus has also been framed in such a way that the basic skills of subject are taught to the students, and everyone might not need to go for higher studies and the scope of securing a job after graduation will increase. It is essential that Biochemistry students select their general electives courses from Chemistry, Physics, Mathematics and/or any branch of Life Sciences disciplines. While the syllabus is in compliance with UGC model curriculum, it is necessary that Biochemistry students should learn “Basic Microbiology” as one of the core courses rather than as elective while. Course on “Concept of Genetics” has been moved to electives. Also, it is recommended that two elective courses namely Nutritional Biochemistry and Advanced Biochemistry may be made compulsory. 2 Type of Courses Number of Courses Course type Description B. -

GANPAT UNIVERSITY FACULTY of SCIENCE TEACHING and EXAMINATION SCHEME Programme Bachelor of Science Branch/Spec
GANPAT UNIVERSITY FACULTY OF SCIENCE TEACHING AND EXAMINATION SCHEME Programme Bachelor of Science Branch/Spec. Microbiology Semester I Effective from Academic Year 2015- Effective for the batch Admitted in June 2015 16 Teaching scheme Examination scheme (Marks) Subject Subject Name Credit Hours (per week) Theory Practical Code Lecture(DT) Practical(Lab.) Lecture(DT) Practical(Lab.) CE SEE Total CE SEE Total L TU Total P TW Total L TU Total P TW Total UMBA101FOM FUNDAMENTALS 04 - 04 - - - 04 - 04 - - - 40 60 100 - - - OF MICROBIOLOGY UCHA101GCH GENERAL 04 - 04 - - - 04 - 04 - - - 40 60 100 - - - CHEMISTRY-I UPHA101GPH GENERAL 04 - 04 - - - 04 - 04 - - - 40 60 100 - - - PHYSICS-I UENA101ENG ENGLISH-I 02 - 02 - - - 02 - 02 - - - 40 60 100 - - - OPEN SUBJECT – 1 02 - 02 - - - 02 - 02 - - - 40 60 100 - - - UPMA101PRA PRACTICAL - - - 02 - 02 - - - 04 - 04 - - - - 50 50 MODULE-I UPCA101PRA PRACTICAL - - - 02 - 02 - - - 04 - 04 - - - - 50 50 MODULE-I UPPA101PRA PRACTICAL - - - 02 - 02 - - - 04 - 04 - - - - 50 50 MODULE-I Total 16 - 16 06 - 06 16 - 16 12 - 12 200 300 500 - 150 150 GANPAT UNIVERSITY FACULTY OF SCIENCE Programme Bachelor of Science Branch/Spec. Microbiology Semester I Version 1.0.1.0 Effective from Academic Year 2015-16 Effective for the batch Admitted in July 2015 Subject code UMBA 101 Subject Name FUNDAMENTALS OF MICROBIOLOGY FOM Teaching scheme Examination scheme (Marks) (Per week) Lecture(DT) Practical(Lab.) Total CE SEE Total L TU P TW Credit 04 -- -- -- 04 Theory 40 60 100 Hours 04 -- -- -- 04 Practical -- -- -- Pre-requisites: Students should have basic knowledge of Microorganisms and microscopy of 10+2 level. Learning Outcome: The course will help the student to understand basic fundamentals of Microbiology and history of Microbiology. -

6. Stellar Spectra
6. Stellar spectra excitation and ionization, Saha’s equation stellar spectral classification Balmer jump, H- 1 Occupation numbers: LTE case Absorption coefficient: κν = niσν à calculation of occupation numbers needed LTE each volume element in thermodynamic equilibrium at temperature T(r) hypothesis: electron-ion collisions adjust equilibrium difficulty: interaction with non-local photons LTE is valid if effect of photons is small or radiation field is described by Planck function at T(r) otherwise: non-LTE 2 Excitation in LTE Boltzmann excitation equation nij: number density of atoms in excited level i of ionization stage j (ground level: i=1 neutral: j=0) gij: statistical weight of level i = number of degenerate states Eij excitation energy relative to ground state 2 gij = 2i for hydrogen = (2S+1) (2L+1) in L-S coupling The fraction relative to the total number of atoms of in ionization stage j is Uj (T) is called the partition function 3 Ionization in LTE: Saha’s formula Generalize Boltzmann equation for ratio of two contiguous ionic species j and j+1 Consider ionization process j à j+1 initial state: n1j & statistical weight g1j final state: n1j+1 + free electron & statistical weight g1j+1 gEl number of ions in groundstate with free electron with velocity in (v,v+dv) in phase space 3 gEl: volume in phase space normalized to smallest possible volume (h ) for electron: 2 spin orientations 4 Ionization: Saha’s formula using Boltzmann formula Sum over all final states: integrate over all phase space - ionization falls with n (recombinations) -

Chemistry in One Dimension Pierre-Fran¸Coisloos,1, A) Caleb J
Chemistry in One Dimension Pierre-Fran¸coisLoos,1, a) Caleb J. Ball,1 and Peter M. W. Gill1, b) Research School of Chemistry, Australian National University, Canberra ACT 0200, Australia We report benchmark results for one-dimensional (1D) atomic and molecular sys- tems interacting via the Coulomb operator jxj−1. Using various wavefunction-type approaches, such as Hartree-Fock theory, second- and third-order Møller-Plesset per- turbation theory and explicitly correlated calculations, we study the ground state of atoms with up to ten electrons as well as small diatomic and triatomic molecules containing up to two electrons. A detailed analysis of the 1D helium-like ions is given and the expression of the high-density correlation energy is reported. We report the total energies, ionization energies, electron affinities and other interesting properties of the many-electron 1D atoms and, based on these results, we construct the 1D analog of Mendeleev's periodic table. We find that the 1D periodic table contains only two groups: the alkali metals and the noble gases. We also calculate the dissociation + 2+ 3+ curves of various 1D diatomics and study the chemical bond in H2 , HeH , He2 , + 2+ H2, HeH and He2 . We find that, unlike their 3D counterparts, 1D molecules are + primarily bound by one-electron bonds. Finally, we study the chemistry of H3 and we discuss the stability of the 1D polymer resulting from an infinite chain of hydrogen atoms. PACS numbers: 31.15.A-, 31.15.V-, 31.15.xt Keywords: one-dimensional atom; one-dimensional molecule; one-dimensional polymer; helium-like ions arXiv:1406.1343v1 [physics.chem-ph] 5 Jun 2014 a)Electronic mail: Corresponding author: [email protected] b)Electronic mail: [email protected] 1 I. -

Laser Cooling and Slowing of a Diatomic Molecule John F
Abstract Laser cooling and slowing of a diatomic molecule John F. Barry 2013 Laser cooling and trapping are central to modern atomic physics. It has been roughly three decades since laser cooling techniques produced ultracold atoms, leading to rapid advances in a vast array of fields and a number of Nobel prizes. Prior to the work presented in this thesis, laser cooling had not yet been extended to molecules because of their complex internal structure. However, this complex- ity makes molecules potentially useful for a wide range of applications. The first direct laser cooling of a molecule and further results we present here provide a new route to ultracold temperatures for molecules. In particular, these methods bridge the gap between ultracold temperatures and the approximately 1 kelvin temperatures attainable with directly cooled molecules (e.g. with cryogenic buffer gas cooling or decelerated supersonic beams). Using the carefully chosen molecule strontium monofluoride (SrF), decays to unwanted vibrational states are suppressed. Driving a transition with rotational quantum number R=1 to an excited state with R0=0 eliminates decays to unwanted ro- tational states. The dark ground-state Zeeman sublevels present in this specific scheme are remixed via a static magnetic field. Using three lasers for this scheme, a given molecule should undergo an average of approximately 100; 000 photon absorption/emission cycles before being lost via un- wanted decays. This number of cycles should be sufficient to load a magneto-optical trap (MOT) of molecules. In this thesis, we demonstrate transverse cooling of an SrF beam, in both Doppler and a Sisyphus-type cooling regimes. -

Importance of Impedance Spectroscopy Technique in Materials Characterization: a Brief Review M Joshi
Importance of Impedance Spectroscopy Technique in Materials Characterization: A Brief Review M Joshi To cite this version: M Joshi. Importance of Impedance Spectroscopy Technique in Materials Characterization: A Brief Review . Mechanics, Materials Science & Engineering MMSE Journal. Open Access, 2017, 9, 10.2412/mmse.42.57.345. hal-01504661 HAL Id: hal-01504661 https://hal.archives-ouvertes.fr/hal-01504661 Submitted on 10 Apr 2017 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution| 4.0 International License Mechanics, Materials Science & Engineering, April 2017 – ISSN 2412-5954 Importance of Impedance Spectroscopy Technique in Materials Characterization: A Brief Review47 M.J. Joshi1 1 – Crystal Growth Laboratory, Physics Department, Saurashtra University, Rajkot, India DOI 10.2412/mmse.42.57.345 provided by Seo4U.link Keywords: Nyquist plot, Grain and Grain Boundary Effect, gel growth, slow evaporation method, nanoparticles. ABSTRACT. Impedance spectroscopy is a popular analytical tool in materials research and gives plenty of information after careful analysis. Experimentally obtained data can be analyzed by using a mathematical model based on possible physical theory that predicts theoretical impedance or a relatively empirical equivalent circuit. -

GUMBOS As Matrices for Matrix-Assisted Laser Desorption
Louisiana State University LSU Digital Commons LSU Doctoral Dissertations Graduate School 2015 GUMBOS as Matrices for Matrix-Assisted Laser Desorption Ionization Time of Flight Mass Spectrometry Hashim Abdullah Al Ghafly Louisiana State University and Agricultural and Mechanical College, [email protected] Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_dissertations Part of the Chemistry Commons Recommended Citation Al Ghafly, Hashim Abdullah, "GUMBOS as Matrices for Matrix-Assisted Laser Desorption Ionization Time of Flight Mass Spectrometry" (2015). LSU Doctoral Dissertations. 3253. https://digitalcommons.lsu.edu/gradschool_dissertations/3253 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Doctoral Dissertations by an authorized graduate school editor of LSU Digital Commons. For more information, please [email protected]. GUMBOS AS MATRICES FOR MATRIX-ASSISTED LASER DESORPTION IONIZATION TIME OF FLIGHT MASS SPECTROMETRY A Dissertation Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Doctor of Philosophy in The Department of Chemistry by Hashim Al Ghafly B.S., King Saudi University Riyadh, 1997 M.S., University of Nebraska Omaha, 2009 May 2015 To my Parents Abdullah Al Ghafly and Norah Bouhlaqa To my children, my wife and my friends for their continuous love, encouragement and support throughout the years. ii ACKNOWLEDGEMENTS I deeply appreciate the excellent guidance, dedicated teaching, constant encouragement, kind assistance, and the precious time devoted by my supervisor, Professor Isiah M. Warner. I am confident that the experience I gained in the Warner lab will tremendously help me to succeed in my future endeavors.