Synthesis and Analysis of UV-Filters Intercalated in Zeolite L
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Thesis Entitled Evaluating UVB and UVA Boosting Technologies For
A Thesis entitled Evaluating UVB and UVA Boosting Technologies for Chemical and Physical Sunscreens by An Ngoc Hiep Huynh Submitted to the Graduate Faculty as partial fulfillment of the requirements for the Master of Science Degree in Pharmaceutical Sciences Industrial Pharmacy ___________________________________________ Gabriella Baki, Ph.D., Committee Chair ___________________________________________ Jerry Nesamony, Ph.D., Committee Member ___________________________________________ Matthew W. Liberatore, Ph.D., Committee Member ___________________________________________ Dr. Amanda C. Bryant-Friedrich, Dean College of Graduate Studies The University of Toledo May 2020 Copyright 2020 An Ngoc Hiep Huynh This document is copyrighted material. Under copyright law, no parts of this document may be reproduced without the expressed permission of the author. An Abstract of Evaluating UVB and UVA Boosting Technologies for Chemical and Physical Sunscreens by An Ngoc Hiep Huynh Submitted to the Graduate Faculty as partial fulfillment of the requirements for the Master of Science Degree in Pharmaceutical Sciences Industrial Pharmacy The University of Toledo May 2020 There are currently 14 organic and 2 inorganic UV filters approved in the United States. Due to coral reef safety concerns, octinoxate and oxybenzone have been banned in Hawaii, Key West, FL and the US Virgin Islands; and octocrylene is also being studied for its potential impact on coral reef safety, leaving 11 organic UV filters as viable options for sunscreen manufacturers – with limitations on their combination. Since consumers are always looking for sunscreens with high SPF and broad-spectrum protection, the need for UVB and UVA protection boosting technologies is greater than ever. In a preliminary study, about two dozen emollients were scanned for their SPF boosting capability with selected organic UV filters. -

GAO-18-61, SUNSCREEN: FDA Reviewed Applications For
United States Government Accountability Office Report to Congressional Committees November 2017 SUNSCREEN FDA Reviewed Applications for Additional Active Ingredients and Determined More Data Needed GAO-18-61 November 2017 SUNSCREEN FDA Reviewed Applications for Additional Active Ingredients and Determined More Data Needed Highlights of GAO-18-61, a report to congressional committees Why GAO Did This Study What GAO Found Using sunscreen as directed with other The Food and Drug Administration (FDA), within the Department of Health and sun protective measures may help Human Services, implemented requirements for reviewing applications for reduce the risk of skin cancer—the sunscreen active ingredients within time frames set by the Sunscreen Innovation most common form of cancer in the Act, which was enacted in November 2014. For example, the agency issued a United States. In the United States, guidance document on safety and effectiveness testing in November 2016. sunscreen is considered an over-the- counter drug, which is a drug available As of August 2017, all applications for sunscreen active ingredients remain to consumers without a prescription. pending after the agency determined more safety and effectiveness data are Some sunscreen active ingredients not needed. By February 2015, FDA completed its initial review of the safety and currently marketed in the United States effectiveness data for each of the eight pending applications, as required by the have been available in products in act. FDA concluded that additional data are needed to determine that the other countries for more than a ingredients are generally recognized as safe and effective (GRASE), which is decade. Companies that manufacture needed so that products using the ingredients can subsequently be marketed in some of these ingredients have sought the United States without FDA’s premarket approval. -

Octinoxate, Octisalate, Avobenzone, Ensulizole, Homosalate
TONYMOLY MAGIC FOOD MANGO MILD SUN BLOCK- octinoxate, octisalate, avobenzone, ensulizole, homosalate cream TONYMOLY CO.,LTD Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies. ---------- ACTIVE INGREDIENT Active ingredients: Ethylhexyl Methoxycinnamate 6.9%, Ethylhexyl Salicylate 4.5%, Butyl Methoxydibenzoylmethane 3.5%, Phenylbenzimidazole Sulfonic Acid 3.5%, Homosalate 3.0% INACTIVE INGREDIENT Inactive ingredients: Water, Butylene Glycol, Alcohol Denat., Octocrylene, Phenethyl Benzoate, Aminomethyl Propanol, Bis-Ethylhexyloxyphenol Methoxyphenyl Triazine, Triceteareth-4 Phosphate, Glycol Stearate, Ammonium Acryloyldimethyltaurate/VP Copolymer, Carbomer, PEG-2 Stearate, Fragrance(Parfum), Phenoxyethanol, Glyceryl Caprylate, Caprylyl Glycol, Mangifera Indica (Mango) Seed Butter, Disodium EDTA, Citrus Limon (Lemon) Fruit Extract, Musa Sapientum (Banana) Fruit Extract, Propylene Glycol, 1,2-Hexanediol, Citrus Aurantium Dulcis (Orange) Fruit Extract, Mangifera Indica (Mango) Fruit Extract, Psidium Guajava Fruit Extract, Citrus Paradisi (Grapefruit) Fruit Extract, Cocos Nucifera (Coconut) Fruit Extract, Actinidia Chinensis (Kiwi) Fruit Extract, Carica Papaya (Papaya) Fruit Extract, Ethylhexylglycerin PURPOSE Purpose: Sunscreen WARNINGS Warnings: For external use only Do not use on damaged or broken skin Stop use and ask a doctor if irritation occurs Keep out of reach of children DESCRIPTION Uses: - helps prevent sunburn - If used as directed with other sun protection measures Directions: decreases the risk of skin cancer and early skin aging caused by the sun Directions: For sunscreen use: - apply liberally 15 minutes before sun exposure - use a water resistant sunscreen if swimming or sweating - reapply at least every 2 hours - Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. -

Research Article Development and Validation of a Stability Indicating
Hindawi Publishing Corporation ISRN Chromatography Volume 2013, Article ID 506923, 12 pages http://dx.doi.org/10.1155/2013/506923 Research Article Development and Validation of a Stability Indicating RP-HPLC Method for the Determination of Two Sun Protection Factors (Koptrizon and Tinosorb S) in Topical Pharmaceutical Formulations Using Experimental Designs Chinmoy Roy1,2 and Jitamanyu Chakrabarty2 1 Analytical Research and Development, Dr. Reddy’s Laboratories Ltd., Bachupally, Hyderabad, Andhra Pradesh 500090, India 2 Department of Chemistry, National Institute of Technology, Durgapur, West Bengal 713209, India Correspondence should be addressed to Chinmoy Roy; [email protected] Received 11 March 2013; Accepted 15 April 2013 Academic Editors: C. Akbay and J. A. P. Coelho Copyright © 2013 C. Roy and J. Chakrabarty. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. A novel, simple, validated stability indicating HPLC method was developed for determination of Koptrizon and Tinosorb S. Stability indicating power of the method was established by forced degradation study. The chromatographic separation was achieved with Waters X Bridge C18 column, by using mobile phase consisting of a mixture of acetonitrile : tetrahydrofuran : water (38 : 38 : 24, v/v/v). The method fulfilled validation criteria and was shown to be sensitive, with limits of detection (LOD) and quantitation −1 −1 (LOQ) of 0.024 and 0.08 gmL for Koptrizon and 0.048 and 0.16 gmL for Tinosorb S, respectively. The developed method is validated for parameters like precision, accuracy, linearity, solution stability, specificity, and ruggedness as per ICH norms. -
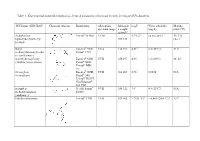
Table 1 - Experimental and Predicted Physical-Chemical Parameters of the Most Recently Investigated UV-Absorbers
Table 1 - Experimental and predicted physical-chemical parameters of the most recently investigated UV-absorbers. INCI name (INN/XAN) Chemical structure Brand name Absorption Molecula LogP Water solubility Melting spectrum range r weight (mg/L) point (°C) (g/mol)4 diethylamino Uvinul® A Plus UVA1 5.7-6.21 <0.01 (20°C) 1 54; 314 hydroxybenzoyl hexyl 397.515 (dec.) 1 benzoate Butyl Eusolex® 9020, UVA 310.393 4.514 2.2 (25°C)4 83.54 methoxydibenzoylmetha Parsol® 1789 ne (avobenzone) 4-methylbenzylidene Eusolex® 6300 UVB 258.397 4.95 1.3 (20°C) 66–68 camphor (enzacamene) Parsol® 5000 Uvinul® MBC 95 Octocrylene Eusolex® OCR, UVB 361.485 6.783 0.00383 N/A (octocrilene) Parsol® 340, Uvinul® N539T, NeoHeliopan® 303 USP isoamyl p- Neo Heliopan® UVB 248.322 3.61 4.9 (25°C)1 N/A methoxycinnamate E1000 (amiloxate) Ethylhexyl triazone Uvinul® T150 UVB 823.092 > 7(20 °C) 6 < 0.001 (20.0 °C) 6 1296 Ethylhexyl Parsol® MCX, UVB 290.403 6.14 0.041 (24 °C and N/A methoxycinnamate Heliopan® New pH 7.1) 4 (octinoxate) Ethylhexyl dimethyl Escalol™ 507 UVB 277.4084 5.774 0.54 (25 °C) 4 N/A PABA (padimate-O) Arlatone 507 Eusolex 6007 benzophenone-3 Eusolex® 4360 UVA2+ UVB 228.247 3.72 3.7 (20°C) 2 62-652 (oxybenzone) bis-ethylhexyloxyphenol Tinosorb® S UVA1+UVB 627.826 12.61 <10-4 80.401 methoxyphenol triazine (bemotrizinol) Phenylbenzimidazole Eusolex® 232 UVA2+ UVB 274.2945 -1.1 (pH 5) > 30% (As sodium N/A sulfonic acid Parsol® HS -2.1 (pH 8)5 or (ensulizole) Neo Heliopan® triethanolammoniu Hydro m salt at 20°C) 5 1 (3) 2 (34) 3 (44) 4 Pubchem 5 SCCP/1056/06 Opinion on phenylbenzimidazole sulfonic acid and its salts 6 BASF safety data sheet Table 2 – In vitro studies for the assessment of skin permeation/penetration of sunscreens. -

Pdf; Chi 2015 DPP Air in Cars.Pdf; Dodson 2014 DPP Dust CA.Pdf; Kasper-Sonnenberg 2014 Phth Metabolites.Pdf; EU Cosmetics Regs 2009.Pdf
Bouge, Cathy (ECY) From: Nancy Uding <[email protected]> Sent: Friday, January 13, 2017 10:24 AM To: Steward, Kara (ECY) Cc: Erika Schreder Subject: Comments re. 2016 CSPA Rule Update - DPP Attachments: DPP 131-18-0 exposure.pdf; Chi 2015 DPP air in cars.pdf; Dodson 2014 DPP dust CA.pdf; Kasper-Sonnenberg 2014 phth metabolites.pdf; EU Cosmetics Regs 2009.pdf Please accept these comments from Toxic-Free Future concerning the exposure potential of DPP for consideration during the 2016 CSPA Rule update. Regards, Nancy Uding -- Nancy Uding Grants & Research Specialist Toxic-Free Future 206-632-1545 ext.123 http://toxicfreefuture.org 1 JES-00888; No of Pages 9 JOURNAL OF ENVIRONMENTAL SCIENCES XX (2016) XXX– XXX Available online at www.sciencedirect.com ScienceDirect www.elsevier.com/locate/jes Determination of 15 phthalate esters in air by gas-phase and particle-phase simultaneous sampling Chenchen Chi1, Meng Xia1, Chen Zhou1, Xueqing Wang1,2, Mili Weng1,3, Xueyou Shen1,4,⁎ 1. College of Environmental & Resource Sciences, Zhejiang University, Hangzhou 310058, China 2. Zhejiang National Radiation Environmental Technology Co., Ltd., Hangzhou 310011, China 3. School of Environmental and Resource Sciences, Zhejiang Agriculture and Forestry University, Hangzhou 310058, China 4. Zhejiang Provincial Key Laboratory of Organic Pollution Process and Control, Hangzhou 310058, China ARTICLE INFO ABSTRACT Article history: Based on previous research, the sampling and analysis methods for phthalate esters (PAEs) Received 24 December 2015 were improved by increasing the sampling flow of indoor air from 1 to 4 L/min, shortening the Revised 14 January 2016 sampling duration from 8 to 2 hr. -

FDA Proposes Sunscreen Regulation Changes February 2019
FDA Proposes Sunscreen Regulation Changes February 2019 The U.S. Food and Drug Administration (FDA) regulates sunscreens to ensure they meet safety and eectiveness standards. To improve the quality, safety, and eectiveness of sunscreens, FDA issued a proposed rule that describes updated proposed requirements for sunscreens. Given the recognized public health benets of sunscreen use, Americans should continue to use broad spectrum sunscreen with SPF 15 or higher with other sun protective measures as this important rulemaking eort moves forward. Highlights of FDA’s Proposals Sunscreen active ingredient safety and eectiveness Two ingredients (zinc oxide and titanium dioxide) are proposed to be safe and eective for sunscreen use and two (aminobenzoic acid (PABA) and trolamine salicylate) are 1 proposed as not safe and eective for sunscreen use. FDA proposes that it needs more safety information for the remaining 12 sunscreen ingredients (cinoxate, dioxybenzone, ensulizole, homosalate, meradimate, octinoxate, octisalate, octocrylene, padimate O, sulisobenzone, oxybenzone, avobenzone). New proposed sun protection factor Sunscreen dosage forms (SPF) and broad spectrum Sunscreen sprays, oils, lotions, creams, gels, butters, pastes, ointments, and sticks are requirements 2 proposed as safe and eective. FDA 3 • Raise the maximum proposed labeled SPF proposes that it needs more data for from SPF 50+ to SPF 60+ sunscreen powders. • Require any sunscreen SPF 15 or higher to be broad spectrum • Require for all broad spectrum products SPF 15 and above, as SPF increases, broad spectrum protection increases New proposed label requirements • Include alphabetical listing of active ingredients on the front panel • Require sunscreens with SPF below 15 to include “See Skin Cancer/Skin Aging alert” on the front panel 4 • Require font and placement changes to ensure SPF, broad spectrum, and water resistance statements stand out Sunscreen-insect repellent combination 5 products proposed not safe and eective www.fda.gov. -

Chemical UVR Absorbers
Chemical UVR Absorbers The names given in bold and used Diisopropyl methyl cinnamate Glyceryl ethyihexanoate dimethoxy- throughout this handbook are those of Empirical formula: cinnamate the International Nomenclature of C 6H22O2 Chemical names. Cosmetic Ingredients. Glyceryl octanoate dimethoxycinnamate; Chemical names: 2-propenoic acid, 3-(4-methoxyphenyl)-, 2-Propenoic acid, 3-12,4bis(1 diester with 1 ,3-dihydroxy-2-(2-ethyl-1 - methylethyphenyl-methyl ester; 2,5- oxohexyl)oxypropane diisopropyl methyl cinnamate _ lsoamyl-para-methoxycinnamate Ethyihexyl methoxycinnamate Empirical formula: Empirical formula: C151-12003 C 8H26O3 Chemical names: Cinnamates Chemical names: Amyl4-methoxycinnamate; isopentyl-4- 2-Ethylhexyl-4-methoxycin nam ate; methoxycinnamate; isopenlyl-para- Cinoxate 2-ethyl-hexyl-para-methoxycinnamate; methoxy-cinnamate; 3-(4-methoxyphenyl)- Empirical formula: para-methoxycinnamic acid, 2-ethylhexyl 2-propenoic acid, isopentyl ester Ci4HieO4 ester; 3-(4-methoxyphenyl)-2-propenoic acid, 2-ethylhexyl ester; octinoxate; octyl Trade names: Chemical names: methoxycinnamate; 2-propenoic acid, 3- Neo Heliopan type E 1000; Solarum AMC 2- Ethoxyothyl-para-methoxyci n nam ate; (4-methoxyphenyl)-2-ethylhexyl ester 2-propenoic acid, 3-(4-methoxyphery- para-A minobenzoic acids (PA BAs) 2-ethoxyethyl ester; 2-ethoxyethyl-4- Trade names: methoxycinnamate AEC Octyl Methoxycinnamate; Escalol Amyl dimethyl FABA 557; Eusolex 2292; Heliosol 3; Empirical formula: Trade names: Jeescreen OMC; Katoscreen OMC; Nec C14H21 NO2 Giv Tan F; Phiasol -

WO 2013/036901 A2 14 March 2013 (14.03.2013) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2013/036901 A2 14 March 2013 (14.03.2013) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61K 8/30 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (21) International Application Number: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, PCT/US2012/054376 DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, 10 September 2012 (10.09.2012) KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, (25) Filing Language: English NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, (26) Publication Language: English RW, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, (30) Priority Data: ZM, ZW. 61/532,701 9 September 201 1 (09.09.201 1) US (84) Designated States (unless otherwise indicated, for every (71) Applicant (for all designated States except US): UNIVER¬ kind of regional protection available): ARIPO (BW, GH, SITY OF FLORIDA RESEARCH FOUNDATION, GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, SZ, TZ, INC. -

Les Filtres UV Dans Les Cosmétiques : Une Présence Obligatoire ?
UNIVERSITÉ DE NANTES UFR SCIENCES PHARMACEUTIQUES ET BIOLOGIQUES ____________________________________________________________________________ ANNÉE 2015 N° THÈSE pour le DIPLÔME D’ÉTAT DE DOCTEUR EN PHARMACIE par Anouk POCHAT Présentée et soutenue publiquement le 14 décembre 2015 Les filtres UV dans les cosmétiques : une présence obligatoire ? Président : Mme Laurence Coiffard, Professeur des universités, Laboratoire de Pharmacie industrielle et Cosmétologie Membres du jury : Directeur de thèse : Mme Céline Couteau, Maître de conférences, HDR, Laboratoire de Pharmacie industrielle et Cosmétologie Mme Françoise PEIGNE , Maitre de conférences à la retraite Page 1 Remerciements A mon président de jury, Professeur à la faculté des sciences pharmaceutiques de Nantes J’exprime mes profonds remerciements à Mme Coiffard, pour m’avoir fait l’honneur de présider mon jury de thèse. A mon directeur de thèse, Maître de conférences à la faculté de Pharmacie de Nantes Je remercie Mme Couteau pour m’avoir conseillée et guidée tout au long de mon travail. A Madame Françoise PEIGNE, Docteur en Pharmacie, Je remercie Mme Peigné d’avoir accepté d’assister à ma soutenance. A ma mère, Je te remercie de m’avoir soutenue et encouragée tout au long de mes études. A mon conjoint, Je te remercie pour ta patience, ton écoute et ton soutien. A mes frères, Je vous remercie pour vos encouragements Page 2 I.Introduction Une exposition prolongée aux UVA et aux UVB peut avoir de graves conséquences sur la santé comme, par exemple, la survenue de cancers cutanés (Aubin F., 2001). Les filtres UV permettent d’assurer une protection dans les domaines UVA et/ou UVB. On en trouve dans les produits de protection solaire que le public utilise ponctuellement lors des expositions prolongées au soleil. -

OSEQUE ZSOLE DUAL SUN BLOCK- Octinoxate Avobenzone Bisoctrizole Spray SONGHAK CO., LTD
OSEQUE ZSOLE DUAL SUN BLOCK- octinoxate avobenzone bisoctrizole spray SONGHAK CO., LTD. ---------- Active ingredients: Octyl Methoxycinnamate 7.5%, Ethylhexyl Salicylate 5%, Avobenzone 2%, Bisoctrizole 0.5% Purpose: Sunscreen Inactive Ingredients: Water, Alcohol Denat, Butylene Glycol, Glycerin, Bis-Ethylhexyloxyphenol Methoxyphenyl Triazine, Niacinamide, Isononyl Isononanoate, Glacier Water, Butyloctyl Salicylate, Xanthan Gum, Decyl Glucoside, Disodium EDTA, Methylparaben, Propylparaben, Fragrance Do not use: on wounds, rashes, dermatitis or damaged skin Keep out of reach of children. In case of accidental ingestion, seek professional assistance or contact a Poison Control Center immediately Stop use: Please stop using this product and contact a dermatologist 1. If allergic reaction or irritation occurs 2. If direct sunlight affects the area as above When using this product: Do not use other than directed Warnings: For external use only. Not to be swallowed. Avoid contact with eyes. Discontinue use if signs of irritation or rash appear Uses: Spray or apply liberally over the face and body 15 minutes prior to sun exposure. Always ensure total coverage of all sun exposed areas. Re-apply every 1-2 hours and always after swimming. For spray type, shake it well before use and spray it evenly at 20cm-30cm apart from face or wherever needed. For spray type - shake it well before use For lotion type - turn a cap to use Storage: Keep in a dry or room temperature area OSEQUE ZSOLE DUAL SUN BLOCK octinoxate avobenzone bisoctrizole spray Product -

Annex VI, Last Update: 02/08/2021
File creation date: 03/10/2021 Annex VI, Last update: 22/09/2021 LIST OF UV FILTERS ALLOWED IN COSMETIC PRODUCTS Substance identification Conditions Wording of Reference Maximum conditions of Product Type, concentration Update date number Chemical name / INN / XAN Name of Common Ingredients Glossary CAS Number EC Number Other use and body parts in ready for use warnings preparation 2 N,N,N-Trimethyl-4-(2-oxoborn-3-ylidenemethyl CAMPHOR BENZALKONIUM 52793-97-2 258-190-8 6% 15/10/2010 ) anilinium methyl sulphate METHOSULFATE 3 Benzoic acid, 2-hydroxy-, HOMOSALATE 118-56-9 204-260-8 10% 02/08/2021 3,3,5-trimethylcyclohexyl ester / Homosalate 4 2-Hydroxy-4-methoxybenzophenone / BENZOPHENONE-3 131-57-7 205-031-5 6% Reg (EU) Not more than Contains 02/08/2021 Oxybenzone 2017/238 of 10 0,5 % to protect Benzophenone-3 February 2017- product (1) date of formulation application from September 2017 6 2-Phenylbenzimidazole-5-sulphonic acid and its PHENYLBENZIMIDAZOLE SULFONIC 27503-81-7 248-502-0 8%(as acid) 08/03/2011 potassium, sodium and triethanolamine salts / ACID Ensulizole 7 3,3'-(1,4-Phenylenedimethylene) bis TEREPHTHALYLIDENE DICAMPHOR 92761-26-7 / 410-960-6 / - 10%(as acid) 26/10/2010 (7,7-dimethyl-2-oxobicyclo-[2.2.1] SULFONIC ACID 90457-82-2 hept-1-ylmethanesulfonic acid) and its salts / Ecamsule 8 1-(4-tert-Butylphenyl)-3-(4-methoxyphenyl) BUTYL 70356-09-1 274-581-6 5% 15/10/2010 propane-1,3-dione / Avobenzone METHOXYDIBENZOYLMETHANE 9 alpha-(2-Oxoborn-3-ylidene)toluene-4-sulphoni BENZYLIDENE CAMPHOR SULFONIC 56039-58-8 - 6%(as acid)