Catch, Bycatch and Discards of the Galapagos Marine Reserve Small-Scale Handline Fishery
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
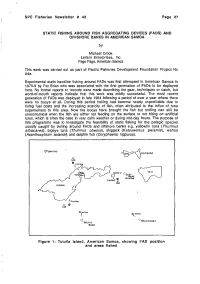
Static Fishing Around Fish Aggregating Devices (Fads) and Offshore Banks
SPC Fisheries Newsletter # 42 Page 27 STATIC FISHING AROUND FISH AGGREGATING DEVICES (FADS) AMD OFFSHORE BANKS IN AMERICAN SAMOA by Michael Crook Leilani Enterprises, Inc Pago Pago, American Samoa This work was carried out as part of Pacific Fisheries Development Foundation Project No 44a. Experimental static handline fishing around FADs was first attempted in American Samoa in 1978-9 by Pat Brian who was associated with the first generation of FADs to be deployed here. No formal reports or records were made describing the gear, techniques or catch, but word-of-mouth reports indicate that this work was mildly successful. The most recent generation of FADs was deployed in late 1984 following a period of over a year where there were no buoys at all. During this period trolling had become nearly unprofitable due to rising fuel costs and the increasing scarcity of fish, often attributed to the influx of tuna superseiners to this area. Now the buoys have brought the fish but trolling can stiil be uneconomical when the fish are either not feeding on the surface or not biting on artificial lures, which is often the case in very calm weather or during mid-day hours. The purpose of this programme was to investigate the feasibility of static fishing for the pelagic species usually caught by trolling around FADs and offshore banks e.g. yellowfin tuna (Thunnus albacares), bigeye tuna (Thunnus obesus), skipjack {Katsuwonus pelamis), wahoo (Acanthocybium solandn) and dolphin fish (Coryphaena hippurus). (P SWAINS a lORl^C VOLOSEGA 'E' FAD n 3 miles TA U /-'^Paao sJ AUNU'U V C~?ago| ^o BANK \ , \_^J 20 miles \ / 5 miles" ^ FAD y TUTUILA w^-> ( 3 miles [ 'c' 0 FAD 35 miles 40 miles "SOUTHEAST " SOUTH BANK Figure 1: Tutuila Island, American Samoa, showing FAD position and areas fished PSger?28! SPCi FisherJesrvWews letters #42; Mettibds (arM ;:inaterialsn Oi'NTASSflSDA n&''~\ mtU^/i Slli'^m-i orh"-t The sixteen trips made between December 1984 and April 1985 were aboard the 40 ft. -

Balancing the Risks and the Benefits of Seafood Consumption in Bermuda
This article was downloaded by: [Canadian Research Knowledge Network] On: 30 September 2008 Access details: Access Details: [subscription number 783016891] Publisher Taylor & Francis Informa Ltd Registered in England and Wales Registered Number: 1072954 Registered office: Mortimer House, 37-41 Mortimer Street, London W1T 3JH, UK Food Additives & Contaminants: Part A Publication details, including instructions for authors and subscription information: http://www.informaworld.com/smpp/title~content=t713599661 Balancing the risks and the benefits of local fish consumption in Bermuda É. Dewailly ab; P. Rouja c; R. Dallaire a; D. Pereg a; T. Tucker d; J. Ward c; J. P. Weber b; J. S. Maguire a; P. Julien a a Public Health Research Unit, Laval University Research Centre-CHUL-CHUQ, Québec, Canada b Institut National de Santé Publique du Québec, Québec, Canada c Department of Conservation Services, Government of Bermuda, Bermuda d Bermuda Underwater Exploration Institute, Bermuda First Published on: 25 September 2008 To cite this Article Dewailly, É., Rouja, P., Dallaire, R., Pereg, D., Tucker, T., Ward, J., Weber, J. P., Maguire, J. S. and Julien, P.(2008)'Balancing the risks and the benefits of local fish consumption in Bermuda',Food Additives & Contaminants: Part A, To link to this Article: DOI: 10.1080/02652030802175285 URL: http://dx.doi.org/10.1080/02652030802175285 PLEASE SCROLL DOWN FOR ARTICLE Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf This article may be used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden. -
Fishing Techniques Used Around Fish Aggregation Devices in French Polynesia
SPC/Fisheries 22/WP.14 27 July 1990 ORIGINAL : FRENCH SOUTH PACIFIC COMMISSION TWENTY-SECOND REGIONAL TECHNICAL MEETING ON FISHERIES (Noumea, New Caledonia 6-10 August, 1990) FISHING TECHNIQUES USED AROUND FISH AGGREGATION DEVICES IN FRENCH POLYNESIA by F. Leproux, G. Moarii, S. Yen EVAAM, B.P. 20, Papeete, Tahiti, French Polynesia 643/90 SPC/Fisheries 22/WP.14 Page 1 FISHING TECHNIQUES USED AROUND FISH AGGREGATION DEVICES IN FRENCH POLYNESIA Mooring FADs in French Polynesian waters, which began in June 1981, has had a favourable impact on local fishermen who, although they were sceptical to begin with, have come to use this assistance increasingly, many of them finding it very helpful. The techniques of fishing around FADs have changed over the years and in 1990 four are still currently in use, while new techniques are constantly being developed and taught in order to obtain better yields. 1. FISHING TECHNIQUES 1.1 Handline fishing Many small boats (3 to 6m) do deep fishing near FADs when a school of tuna is present. The handline is made of nylon line of 130 kg and a tuna hook (cf Diagram no. 1, Plate no. 1). The bait used varies with the season: thus "operu" (Decapterus macarellus), "ature" (Selar crumennhialmus), "numa" (Mulloidichihys samoensis), "marara" (Cysselurus simus), and skipjack fillet (Katsuwonus pelamis) are the most commonly used. The technique consists in placing this bait low down in the school. In order to facilitate the drop of the line, a stone is fixed to it temporarily. When the fisherman considers that he has reached the required depth, he gives the line a sharp jerk upwards which releases the stone and also some crumbs which awake the tunas' appetite. -

Growth of the Shortnose Mojarra Diapterus Brevirostris (Perciformes: Gerreidae) in Central Mexican Pacific
Growth of the Shortnose Mojarra Diapterus brevirostris (Perciformes: Gerreidae) in Central Mexican Pacific Crecimiento de la malacapa Diapterus brevirostris (Perciformes: Gerreidae) en el Pacífico centro mexicano Manuel Gallardo-Cabello,1 Elaine Espino-Barr,2* Esther Guadalupe Cabral-Solís,2 Arturo García-Boa2 y Marcos Puente-Gómez2 1 Instituto de Ciencias del Mar y Limnología Universidad Nacional Autónoma de México Av. Ciudad Universitaria 3000, Col. Copilco México, D. F. (C. P. 04360). 2 INAPESCA, CRIP-Manzanillo Playa Ventanas s/n Manzanillo, Colima (C.P. 28200). Tel: (314) 332 3750 *Corresponding author: [email protected] Abstract Resumen Samples of Shortnose Mojarra Diapterus brevi- Se obtuvieron muestras y datos morfométricos rostris were obtained from the commercial catch de 394 individuos de la malacapa Diapterus from April 2010 to July 2012, morphometric brevirostris, de la captura comercial entre abril data of 394 individuals were registered. The de 2010 y julio de 2012. El estudio del creci- growth study entailed two methods: length fre- miento se realizó por dos métodos: análisis de quency analysis and study of sagittae and as- frecuencia de longitud y el estudio de los otoli- terisci otoliths. Both methods identified six age tos sagittae y asteriscus. Ambos métodos iden- groups. Growth parameters of von Bertalanffy’s tificaron seis grupos de edad. Los parámetros equation were determined by Ford-Walford and de crecimiento de la ecuación de von Berta- Gulland methods and by ELEFAN routine ad- lanffy se determinaron con el método de Ford- justment. Both methods gave the same results: Walford y Gulland y por rutina ELEFAN. L∞= 48.61 cm, K= 0.135, to= -0.696. -

SMALL-SCALE FISHERIES in a WARMING OCEAN EXPLORING ADAPTATION to CLIMATE CHANGE Imprint
THIS PROJECT IS CO-FUNDED BY THE EUROPEAN UNION SMALL-SCALE FISHERIES IN A WARMING OCEAN EXPLORING ADAPTATION TO CLIMATE CHANGE Imprint Publisher WWF Germany; International WWF Centre for Marine Conservation, Hamburg Year of 2020 publication Authors Léa Monnier & Didier Gascuel – Agrocampus Ouest (France) Juan José Alava & William Cheung – University of British Columbia (Canada) Maria José Barragán & Jorge Ramírez – Charles Darwin Foundation (Galapagos) Nikita Gaibor – Instituto Nacional de Pesca (Ecuador) Philipp Kanstinger & Simone Niedermueller – Franck Hollander – WWF Germany Editing all authors Coordination Franck Hollander (WWF Germany), Thomas Koeberich (WWF Germany) Contact [email protected], [email protected] Proofreading Jill Bentley Designed by Silke Roßbach, [email protected] Produced by Maro Ballach (WWF Germany) Funded by WWF Germany, University of British Columbia (Canada) Photo credits Title: © Adam Jason Moore / Getty Images Citation Monnier, L., Gascuel, D., Alava, J.J., Barragán, M.J., Gaibor, N., Hollander, F.A., Kanstinger, P., Niedermueller, S., Ramírez, J., & Cheung, W.W.L. 2020. Small-scale fisheries in a warming ocean: exploring adaptation to climate change. Scientific report. WWF Germany. © 2020 WWF Germany, Berlin This study was led by © freepik © Presentation of the project This publication has been produced with the financial contribution of the European Union within the framework of the pan-European awareness-raising project Fish Forward 2. Fish Forward 2 aims to achieve behaviour change of consumers, corporates and authorities in Europe based on an increased awareness and knowledge of the implications of seafood con- sumption and sourcing on people and oceans in the developing countries, but also in Europe. -

Perciformes: Epinephelidae)
NOTE BRAZILIAN JOURNAL OF OCEANOGRAPHY, 57(2):145-147, 2009 FIRST RECORD OF PARTIAL MELANISM IN THE CONEY CEPHALOPHOLIS FULVA (PERCIFORMES: EPINEPHELIDAE) Thiony Simon 1; Jean-Christophe Joyeux 2 and Raphael Mariano Macieira 3 Universidade Federal do Espírito Santo Departamento de Oceanografia e Ecologia - Laboratório de Ictiologia (Av. Fernando Ferrari, 514, 29075-910 Vitória, ES, Brasil) [email protected]; [email protected]; [email protected] Many abnormalities in the coloration of during sampling. None, however, presented any type fishes have been recorded, including albinism, of coloration abnormality. The frequency of melanism and ambicoloration (e.g . DAHLBERG, occurrence of the anomaly was therefore estimated to 1970). Melanism, according to Gould and Pyle (1896), be 0.68 %. The specimen was photographed still fresh is characterized by the presence of an excessive (Fig. 1a) and maintained frozen until fixation in 10 % amount of pigment in tissues and skin. In fishes, formaldehyde and preservation in 70 % ethanol. The melanism may occur in varying degrees of intensity area of the melanic part of the body was estimated (PIGG, 1998) and can, in some cases, result from from a digital photography of the right side of the fish injury (DAHLBERG, 1970), genetic inheritance (Fig. 1a). A 1300-square grid was digitally overlaid (HORTH, 2006), intergeneric hybridization (ELWIN, onto the photography to determine the proportion of 1957) or parasite infestation (HSIAO, 1941). squares over melanic skin. As both sides displayed the The coney Cephalopholis fulva (Linnaeus, some pattern and extent of melanosis, there was no 1758) is distributed in the Western Atlantic from need for measuring the area on the left side, and the South Carolina, USA, to Southeastern Brazil result obtained for the right side was extrapolated for (FIGUEIREDO; MENEZES, 1980). -

Three Simple Rules for High Catches, High Profits and Healthy Ecosystems
Three simple rules for high catches, high profits and healthy ecosystems Position paper for the round-table discussion Towards a Sustainable Fishery Sector Block 2, Interactions between Fisheries and Nature, Wednesday 23 June 2021 by Rainer Froese, GEOMAR, Kiel, Germany, [email protected] Rule 1: Do not take out more than is regrown Taking out more than is regrown is called overfishing and will shrink the fished stock below levels that can produce the maximum longterm catch (MSY). Despite the legal obligation to end overfishing in 2020, the total allowed catches (TACs) for about 40% of the stocks with suitable data in the North Sea and adjacent waters constituted overfishing (1). Overfishing is stupid: with overfishing, more effort = money is spent to get lower catches than possible at lower value and with unnecessarily high impacts on the ecosystem. The lower value is caused by overfishing shrinking the mean size of the fish and smaller fish bring lower price per kg. Impact is unnecessary high because better catches can be obtained from non- overfished stocks with less gear deployment, therefore less by-catch and less physical impact. To end overfishing, catches have to be reduced for 1-4 years, depending on current stock size. The loss caused by such reduced catches is easily regained within a few years after rebuilt stock size allows for permanent high catches at e.g. 90% of the MSY level. The 90% MSY is needed for stability; with fishing at MSY, there is a 50% chance of shrinking the stock below the MSY level and thus reducing future catches. -

Structure and Dynamics of Demersal Assemblages on the Continental Shelf and Upper Slope Off Ghana, West Africa
MARINE ECOLOGY PROGRESS SERIES Vol. 220: 1–12, 2001 Published September 27 Mar Ecol Prog Ser Structure and dynamics of demersal assemblages on the continental shelf and upper slope off Ghana, West Africa Kwame A. Koranteng* Marine Fisheries Research Division, PO Box BT-62, Tema, Ghana ABSTRACT: Using two-way indicator species analysis and detrended correspondence analysis, species on the continental shelf and upper slope of Ghana were classified into 6 assemblages. The structure of the assemblages is determined primarily by depth and type of sediment on the seabed. There are clear faunal discontinuities around 30–40, 100 and 200 m depth. The dynamics of the assemblages are influenced by physico-chemical parameters of the water masses, mainly tempera- ture, salinity and dissolved oxygen, which are periodically modified by the seasonal coastal upwelling that occurs in the area. The observed changes in the composition and relative importance of species in the assemblages can be related to increased fishing activity and environmental forcing. KEY WORDS: Species assemblages · Structure and dynamics · Continental shelf and slope · Ghana Resale or republication not permitted without written consent of the publisher INTRODUCTION nental shelf using data from the Guinean Trawling Survey (Williams 1968). In fisheries, defining the aggregation of species in In the last 3 decades, significant changes have the ecosystem is the basis for managing species by the occurred in the biological and physical components of management unit approach (Tyler et al. 1982). Caddy the Gulf of Guinea marine ecosystem and in nearshore & Sharp (1986) also pointed out that such studies are forcing factors that could have an effect on species necessary to gain a better understanding of multi- aggregations in the sub-region (Koranteng 1998). -

Fish Assemblages Associated with Red Grouper Pits at Pulley Ridge, A
419 Abstract—Red grouper (Epineph- elus morio) modify their habitat by Fish assemblages associated with red grouper excavating sediment to expose rocky pits, providing structurally complex pits at Pulley Ridge, a mesophotic reef in the habitat for many fish species. Sur- Gulf of Mexico veys conducted with remotely op- erated vehicles from 2012 through 2015 were used to characterize fish Stacey L. Harter (contact author)1 assemblages associated with grouper Heather Moe1 pits at Pulley Ridge, a mesophotic 2 coral ecosystem and habitat area John K. Reed of particular concern in the Gulf Andrew W. David1 of Mexico, and to examine whether invasive species of lionfish (Pterois Email address for contact author: [email protected] spp.) have had an effect on these as- semblages. Overall, 208 grouper pits 1 Southeast Fisheries Science Center were examined, and 66 fish species National Marine Fisheries Service, NOAA were associated with them. Fish as- 3500 Delwood Beach Road semblages were compared by using Panama City, Florida 32408 several factors but were considered 2 Harbor Branch Oceanographic Institute to be significantly different only on Florida Atlantic University the basis of the presence or absence 5600 U.S. 1 North of predator species in their pit (no Fort Pierce, Florida 34946 predators, lionfish only, red grou- per only, or both lionfish and red grouper). The data do not indicate a negative effect from lionfish. Abun- dances of most species were higher in grouper pits that had lionfish, and species diversity was higher in grouper pits with a predator (lion- The red grouper (Epinephelus morio) waters (>70 m) of the shelf edge and fish, red grouper, or both). -

Valuable but Vulnerable: Over-Fishing and Under-Management Continue to Threaten Groupers So What Now?
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/339934856 Valuable but vulnerable: Over-fishing and under-management continue to threaten groupers so what now? Article in Marine Policy · June 2020 DOI: 10.1016/j.marpol.2020.103909 CITATIONS READS 15 845 17 authors, including: João Pedro Barreiros Alfonso Aguilar-Perera University of the Azores - Faculty of Agrarian and Environmental Sciences Universidad Autónoma de Yucatán -México 215 PUBLICATIONS 2,177 CITATIONS 94 PUBLICATIONS 1,085 CITATIONS SEE PROFILE SEE PROFILE Pedro Afonso Brad E. Erisman IMAR Institute of Marine Research / OKEANOS NOAA / NMFS Southwest Fisheries Science Center 152 PUBLICATIONS 2,700 CITATIONS 170 PUBLICATIONS 2,569 CITATIONS SEE PROFILE SEE PROFILE Some of the authors of this publication are also working on these related projects: Comparative assessments of vocalizations in Indo-Pacific groupers View project Study on the reef fishes of the south India View project All content following this page was uploaded by Matthew Thomas Craig on 25 March 2020. The user has requested enhancement of the downloaded file. Marine Policy 116 (2020) 103909 Contents lists available at ScienceDirect Marine Policy journal homepage: http://www.elsevier.com/locate/marpol Full length article Valuable but vulnerable: Over-fishing and under-management continue to threaten groupers so what now? Yvonne J. Sadovy de Mitcheson a,b, Christi Linardich c, Joao~ Pedro Barreiros d, Gina M. Ralph c, Alfonso Aguilar-Perera e, Pedro Afonso f,g,h, Brad E. Erisman i, David A. Pollard j, Sean T. Fennessy k, Athila A. Bertoncini l,m, Rekha J. -

The Blue Paradox: Preemptive Overfishing in Marine Reserves
PAPER The blue paradox: Preemptive overfishing in COLLOQUIUM marine reserves Grant R. McDermotta,1,2, Kyle C. Mengb,c,d,1,2, Gavin G. McDonaldb,e, and Christopher J. Costellob,c,d,e aDepartment of Economics, University of Oregon, Eugene, OR 97403; bBren School of Environmental Science & Management, University of California, Santa Barbara, CA 93106; cDepartment of Economics, University of California, Santa Barbara, CA 93106; dNational Bureau of Economic Research, Cambridge, MA 02138; and eMarine Science Institute, University of California, Santa Barbara, CA 93106 Edited by Jane Lubchenco, Oregon State University, Corvallis, OR, and approved July 25, 2018 (received for review March 9, 2018) Most large-scale conservation policies are anticipated or an- This line of reasoning suggests that the anticipation of a new nounced in advance. This risks the possibility of preemptive conservation policy may give rise to a set of incentives that is resource extraction before the conservation intervention goes distinct from—but possibly just as important as—the incentives into force. We use a high-resolution dataset of satellite-based fish- arising from the policy’s implementation. While this kind of pre- ing activity to show that anticipation of an impending no-take emptive behavior has been well-documented for landowners, gun marine reserve undermines the policy by triggering an unin- owners, and owners of natural resource extraction rights, it has tended race-to-fish. We study one of the world’s largest marine not been studied in the commons. For vast swaths of the ocean, reserves, the Phoenix Islands Protected Area (PIPA), and find that no single owner has exclusive rights and so must compete against fishers more than doubled their fishing effort once this area was others for extraction. -

Marine Protected Areas (Mpas) in Management 1 of Coral Reefs
ISRS BRIEFING PAPER 1 MARINE PROTECTED AREAS (MPAS) IN MANAGEMENT 1 OF CORAL REEFS SYNOPSIS Marine protected areas (MPAs) may stop all extractive uses, protect particular species or locally prohibit specific kinds of fishing. These areas may be established for reasons of conservation, tourism or fisheries management. This briefing paper discusses the potential uses of MPAs, factors that have affected their success and the conditions under which they are likely to be effective. ¾ MPAs are often established as a conservation tool, allowing protection of species sensitive to fishing and thus preserving intact ecosystems, their processes and biodiversity and ultimately their resilience to perturbations. ¾ Increases in charismatic species such as large groupers in MPAs combined with the perception that the reefs there are relatively pristine mean that MPAs can play a significant role in tourism. ¾ By reducing fishing mortality, effective MPAs have positive effects locally on abundances, biomass, sizes and reproductive outputs of many exploitable site- attached reef species. ¾ Because high biomass of focal species is sought but this is quickly depleted and is slow to recover, poaching is a problem in most reef MPAs. ¾ Target-species ‘spillover’ into fishing areas is likely occurring close to the MPA boundaries and benefits will often be related to MPA size. Evidence for MPAs acting as a source of larval export remains weak. ¾ The science of MPAs is at an early stage of its development and MPAs will rarely suffice alone to address the main objectives of fisheries management; concomitant control of effort and other measures are needed to reduce fishery impacts, sustain yields or help stocks to recover.